Abstract
The 2017–2018 seasonal influenza epidemics were severe in the US and Australia where the A(H3N2) subtype viruses predominated. Although circulating A(H3N2) viruses did not differ antigenically from that recommended by the WHO for vaccine production, overall interim vaccine effectiveness estimates were below historic averages (33%) for A(H3N2) viruses. The majority (US) or all (Australian) vaccine doses contained multiple amino-acid changes in the hemagglutinin protein, resulting from the necessary adaptation of the virus to embryonated hen’s eggs used for most vaccine manufacturing. Previous reports have suggested a potential negative impact of egg-driven substitutions on vaccine performance. With BARDA support, two vaccines licensed in the US are produced in cell culture: recombinant influenza vaccine (RIV, Flublok™) manufactured in insect cells and inactivated mammalian cell-grown vaccine (ccIIV, Flucelvax™). Quadrivalent ccIIV (ccIIV4) vaccine for the 2017–2018 influenza season was produced using an A(H3N2) seed virus propagated exclusively in cell culture and therefore lacking egg adaptative changes. Sufficient ccIIV doses were distributed (but not RIV doses) to enable preliminary estimates of its higher effectiveness relative to the traditional egg-based vaccines, with study details pending. The increased availability of comparative product-specific vaccine effectiveness estimates for cell-based and egg-based vaccines may provide critical clues to inform vaccine product improvements moving forward.
Similar content being viewed by others
The 2017–2018 influenza epidemic revived public health concerns
The 2017–2018 influenza season in the United States was in many respects the most severe since the A(H1N1) pandemic of 2009. Influenza virus circulation began to increase in early November and then increased rapidly from December through early February. A(H3N2) subtype viruses predominated over influenza B and A(H1N1), until late in the season when influenza B virus activity peaked. The levels of influenza-like illness, which reflects outpatient visits and emergency department visits, were the highest seen in recent influenza seasons including those when A(H3N2) predominated.1 In contrast to previous years, the US 2017–2018 season was characterized by widespread and high intensity virus circulation at the same time in most states2 Cumulative rates of influenza-confirmed hospitalizations exceeded those seen in the 2014–2015 season, an A(H3N2) predominant season categorized as one of high severity.3 In Europe, A(H3N2) viruses also co-circulated in many countries along with A(H1N1) and influenza B viruses, while in the Southern Hemisphere season in Australia A(H3N2) viruses and to a lesser extent B/Yamagata viruses predominated during the biggest influenza season in the last 20 years.4,5,6
Seasonal epidemics dominated by A(H3N2) typically result in higher rates of infection, hospitalization, and death, especially among the elderly compared with outbreaks due to other subtypes. Based on estimates of influenza disease burden, in A(H3N2) epidemics like those in the 2012–2013 and 2014–2015 seasons, Centers for Disease Control and Prevention (CDC) predicted that as many as 35.6 million illnesses, 16.6 million medically attended visits, 710,000 hospitalizations, and 56,000 deaths could result in such seasons.7 Similarly, excess winter mortality in Europe was notably elevated in winter epidemics when influenza A(H3N2) viruses predominated.8
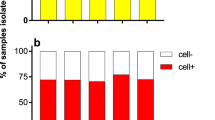
Vaccination remains the best available intervention to prevent morbidity and mortality caused by the four different influenza A and B viruses currently co-circulating in humans. The interim vaccine effectiveness (VE)9 against A(H3N2) during the 2017–2018 season was lower than prior multi-year pooled VE estimates10 in mid-season reports from the Northern Hemisphere, including adults 19–64 years of age from the United States,11 Canada,12 and Europe,13 with similar observations from Australia for the 2017 epidemic season in the Southern Hemisphere.14 Low VE is typically associated with substantial antigenic differences between the vaccine virus and circulating viruses that result from the rapid evolution of co-circulating variants that are selected due to escape from host immunity, as was observed in the 2014–2015 influenza season.15 However, although the co-circulating A(H3N2) subtype viruses that predominated in the 2017–2018 season comprised many HA genetic clades, no significant antigenic drift from the A/Hong Kong/4801/2014-like cell-propagated reference virus was detected,16 indicating an optimal vaccine strain selection. Nevertheless, 36% of A(H3N2) viruses co-circulating in the US from October to early February, showed reduced inhibition by antisera raised to egg-adapted viruses used for production of influenza vaccines in embryonated hen’s eggs.17 A high proportion of A(H3N2) viruses from Europe were also antigenically distinct from the egg-propagated vaccine reference virus.5 Furthermore, only a small proportion of A(H3N2) isolates could be characterized by hemagglutination inhibition tests and had to be analyzed by other methods such as virus neutralization. One of the multiple factors implicated in reduced VE in 2017–2018 are the changes acquired in the viral hemagglutinin (HA), upon isolation, adaptation, and propagation in eggs, which are the base of the manufacturing system currently used globally18 (Table 1).
Influenza vaccines manufactured in eggs for the 2017–2018 season used a seed virus with three amino-acid substitutions in the HA (N96S, L194P, and T160K, which resulted in the loss of a glycosylation at residue 158) that occurred when viruses from humans were isolated and propagated in eggs and one additional substitution at position 225 (Asp to Gly) after virus reassortment in the laboratory, a requirement for large-scale vaccine manufacturing. Although many other egg-adapted viruses were isolated and tested by WHO-CCs they had other HA mutations that dramatically impacted the antigenic properties and these were not taken forward. Several studies have hypothesized that certain egg-adaptive mutations appear to change viral antigenicity and therefore could be partially responsible for the lower than expected VE.12,19 The occurrence of changes to the HA of human influenza viruses propagated in eggs was recognized decades ago but the detrimental impact of these mutations on vaccine antigenicity has largely had been averted by the WHO Collaborating Centers for influenza (WHO-CC) through the identification of optimal candidate vaccine viruses (CVVs) from the available egg-adapted strains.20,21,22 However, the evolution of A(H3N2) subtype viruses in recent years has resulted in viruses that limit the availability of optimal egg-based vaccine strains. This challenge has added further impetus to the expanded utilization of alternative host systems for vaccine manufacturing (cell-based or recombinant protein-based vaccines), which were concurrently being launched to strengthen public health preparedness for seasonal and pandemic influenza emergencies23,24 (Table 1).
Cell-based and recombinant influenza vaccines
Flucelvax™, was initially licensed as an inactivated trivalent cell culture-grown vaccine (ccIIV3) (originally manufactured using egg-derived vaccine seed viruses) propagated in a qualified mammalian cell line (proprietary 33016PF Madin-Darby Canine Kidney (MDCK)) and was licensed in 2012 by Novartis Vaccines and Diagnostics, Inc. (in 2015, bioCSL acquired Novartis’ influenza vaccine assets and created Seqirus, based in London, UK). This trivalent vaccine was upgraded to a quadrivalent vaccine (ccIIV4 (Flucelvax™)) that was licensed by the Food and Drug Administration (FDA) in 2016. However, both these vaccines were produced from egg-derived virus seeds and therefore, mutations associated with adaptation to eggs were likely still present in the vaccine antigen and effectiveness was likely to be similar to a licensed egg-based vaccine.25 In August 2016, Seqirus received FDA supplemental approval for the use of CVVs that had been isolated and propagated in MDCK cells at either of two supporting WHO-CCs (Centers of Disease Control and Prevention in the US and the Victorian Infectious Diseases Reference Laboratory in Australia) for the manufacture of ccIIV4.26,27,28 This enabled the production of completely cell-derived A(H3N2) influenza virus from the initial virus isolation through to the full manufacture of the vaccine. Thus, in the Fall of 2017, for the first time, a seasonal influenza vaccine (Flucelvax Quadrivalent™) was administered to millions of people in the US, which contained a purely mammalian cell culture-derived influenza A(H3N2) virus as one of its four components.26 Preliminary estimates of relative effectiveness conducted by the FDA based on health insurance claims data from the Centers for Medicare Services indicate that ccIIV4 provided a modest improvement over egg-based vaccines in subjects 65 years of age and older.18,29
The use of a completely cell culture-derived virus in a vaccine, which has not been propagated at any stage in eggs, was the fruition of a 10-year effort that began in 2007 (the key personnel involved in this program are listed in the Acknowledgements). The then Novartis Vaccines and Diagnostics (now Seqirus) joined forces with the WHO-CCs in the US (CDC, Atlanta), the Melbourne-based WHO-CC, and subsequently, with the WHO-CCs in London and Tokyo through several research agreements supported in part by the US Department of Health and Human Services (HHS).30 Under these agreements, the Novartis’ proprietary 33016PF MDCK cell line, which had been qualified and used for vaccine manufacturing, was transferred to the respective WHO-CCs, along with specified media and standard operating procedures to enable isolation of influenza viruses in these cells directly from primary patient samples, for further propagation, characterization and eventual disbursement to cell culture-based vaccine manufacturers.31
These early studies showed that the influenza A(H3N2) virus isolation rate in the 33016PF cells was orders of magnitude greater as compared with eggs, while at the same time avoiding the egg adaptation changes of the human influenza viruses that occurs when these viruses are propagated in eggs.32,33,34 The extent to which seasonal vaccines that are fully derived and manufactured in mammalian cell culture will provide improved VE over egg-manufactured influenza vaccines remains to be determined, as definitive comparative field trials have not been reported yet, but animal and human serological testing suggests that mammalian cell-derived antigen could be, at the least, more protective – in certain seasons – particularly with respect to A(H3N2) and B vaccine components.22,35,36,37
In 2013, the FDA-approved Flublok™ (Protein Sciences Corp., recently acquired by Sanofi Pasteur, based in Lyon, France), which is a vaccine composed of only influenza recombinant HA (recombinant influenza vaccine, RIV) that is manufactured using baculovirus overexpression and purification from infected insect cell cultures (Spodoptera frugiperda, fall army worm). RIV is designed to contain the amino-acid sequence of the HA ectodomain of the cell culture-propagated vaccine prototype viruses that are recommended by WHO. In addition, the RIV manufacturing does not include chemical virus inactivation treatment, which may also alter the vaccines’ antigenicity38,39,40 and contains increased concentration of HA protein (threefold) as compared with IIV. In a randomized controlled trial in adults 50 years or older comparing RIV4 with a licensed egg-grown quadrivalent vaccine, RIV4 was 36% more efficacious (95% confidence interval (CI): 14–53%) in preventing RT-PCR confirmed influenza A and 30% more efficacious overall; 80% of the influenza A viruses identified in this 2014–2015 trial were H3N2 strains.41 Unfortunately, it is difficult to differentiate the respective contributions of the vaccine’s higher HA antigen content and/or lack of egg-adaptive changes towards the higher relative efficacy.
Considering the preliminary level of VE evidence and the limitations of observational studies,42 it will be critical to conduct larger and more rigorous trials as soon as possible. Opportunities for future measurements of RIV4 VE are limited by production and distribution levels in the US, which are not high enough to assess its performance through the established effectiveness monitoring platforms.10,43 Even a modest 5–10 percentage point increase in VE would have prevented an additional 494,000–982,000 illnesses and 11,500–22,800 hospitalizations in the 2016–2017 US influenza season.44 Although further studies are needed to evaluate more fully the potential benefits of cell-based vaccine in reducing the burden of influenza disease, in particular due to A(H3N2) viruses, more transformative product development will ultimately be needed to achieve optimal effectiveness of influenza vaccines overall. The interim VE gains achieved through cell-based and/or recombinant manufacturing platforms could be further leveraged in many directions, including: (i) enhancing immune responses and protective efficacy by formulation with adjuvants such as MF59®, as demonstrated by studies with FluAd45; (ii) enhancing immunogenicity and effectiveness by higher antigen dose formulations as shown by studies with Fluzone™ High Dose46,47; (iii) the potential for producing vaccine antigen in tobacco plants (which are awaiting results from pivotal clinical studies)48; (iv) formulating vaccines in which only one [i.e., A(H3N2)] of the three or four components is produced using cell-based or recombinant systems, while others are still produced in eggs.
Monitoring product-specific vaccine effectivess
Observational influenza VE studies are crucial for assessing vaccine performance in “the real world”, informing on vaccine regulation, policy, and product development. However, current data streams supporting observational influenza VE studies do not yield vaccine product-specific information to assess the relative effectiveness of each of the 11 vaccines distributed in the United States in 2017–2018. Although the use of RIV and ccIIV may offer the potential for better protection over traditional egg-based influenza vaccines, sparse utilization of these vaccines to date does not allow robust comparative effectiveness estimates using current observational studies to support preferential use of one injectable influenza vaccine over another.41 The clinical information gap on the relative effectiveness in the current season has become the focus of intense activity. The FDA has recently announced preliminary results of a study to assess relative effectiveness of ccIIV and egg-based vaccines in persons 65 years and older by examining a US national database of insured benefit claims.18,29 Two additional ongoing studies sponsored by BARDA and the US Department of Defense are expected to provide absolute and relative effectiveness data for ccIIV and egg-based vaccines in subjects between the ages of 4 and 64 years or 4 years and above, respectively.
Development of improved seasonal influenza vaccines will depend on innovative clinical studies evaluating differences between product-specific or platform-specific VE to generate evidence to inform manufacturers, regulators, public health policy makers, and consumers. As the list of new vaccines grows, we need to reassess the best methods to monitor VE of new products and to compare products, including expanding current clinical studies and networks, harmonizing data collection to allow combined individual data analyses and meta-analyses, and designing prospective randomized designs to provide a robust foundation to support short- and long-term efforts to reduce the public health impact of seasonal and pandemic influenza.
The role of private–public partnerships
The Pandemic and All-Hazards Preparedness Act promulgated in 200649 authorized the US Department of Health and Human Services (HHS) to support flexible public–private partnerships (PPPs) to achieve pandemic preparedness goals through development of innovative medical products, including vaccines, drugs, respirators, and diagnostics. Seasonal and pandemic influenza prevention and response are inextricably linked, as preparedness for seasonal influenza viruses is the foundation for preparedness for an influenza pandemic. Starting in 2006, HHS awarded several contracts to companies to establish influenza vaccine manufacturing capacity to support pandemic vaccination response capability. Two major thrusts of the program were focused on development of cell-based and recombinant protein-based vaccines by creating the necessary infrastructure.
In 2007, Novartis V&D and the WHO-CC at the CDC signed a Cooperative Research and Development Agreement to support the use of viruses isolated using cell culture to produce the cell-based influenza vaccine. Similar agreements were established between Novartis and the WHO-CCs in London, Melbourne, and Tokyo. These collaborations were aimed at establishing laboratory protocols and workflows to optimize isolation of viruses suitable for development of seed stocks to manufacture vaccines under a quality system in compliance with cGMP FDA guidelines. Since the inception of the collaborative agreements, the WHO-CCs in Atlanta and Melbourne, have isolated and characterized hundreds of viruses to generate the evidence base required to support the recent regulatory approval by the FDA. A range of MDCK and other manufacturing cell lines were tested to identify a single line that was best suited for the primary isolation of influenza viruses from clinical specimens while excluding adventitious agents, preserving genetic and antigenic characteristics of the original isolate, and allowing for sufficient manufacturing yield in cells and eggs.32,33,34 The 33016PF MDCK cell line developed by Novartis Vaccines and Diagnostics was identified as the best suited among the lines that were evaluated. Although the 33016PF MDCK cell line is being used at the CDC and Melbourne WHO-CCs, the Tokyo CC developed its own qualified MDCK cell line to provide CVVs for manufacturers.50 Such collaborations are the most recent examples of the longstanding PPPs, which are necessary to meet the stringent bi-annual timelines for seasonal influenza vaccine campaigns and now have resulted in the licensure of new non-egg-based vaccines. Cell-based and recombinant protein vaccine technologies offer greater flexibility to realize the potential for more effective vaccines than those produced in eggs while possibly supporting accelerated timelines for vaccine composition updates to keep pace with rapid evolution of influenza viruses or to respond to the inevitable future pandemic(s). The PPPs are excellent examples to illustrate the HHS strategy to establish sustainable pandemic preparedness while promoting seasonal vaccine improvement. Despite the foregoing desirable features of cell-based and recombinant vaccines, during the 2017–2018 influenza season these vaccine technologies only represented approximately 13% (ccIIV) and 1% (RIV), respectively, of all vaccine doses distributed in the US43 and broader implementation of these and other new-generation vaccines may not occur until there is a consistent body of evidence supporting their improved VE.
The severity of the 2017–2018 influenza season in North America and elsewhere was greater than expected, even when the predominance of an A(H3N2) subtype was taken into account. Innovative vaccines that have recently become available represent the culmination of decade-long efforts by industry and public partners to diversify influenza vaccine manufacturing platforms. The new egg-independent platforms represent a milestone that is now being realized, as these vaccines have now been given to millions of people in the US.26,41,51,52,53 The culmination of this work for ccIIV4 will be materialized when all four components of the seasonal influenza vaccines are derived from production systems that yield optimal immunogens without host selected mutations. The higher relative efficacy of RIV during 2014–2015, principally on the H3N2 subtype, is encouraging, and additional observations on its relative effectiveness against other viral subtypes and during additional seasons, would be welcome. As each of these practical steps is taken, it is anticipated that there will be a subsequent rise in the effectiveness of seasonal influenza vaccines. However, additional work to understand host-related immune factors such as the impact of prior exposure and immunity to a subsequent vaccine response will also likely lead to strategies that improve influenza VE. While we await the development and availability of the next generation of influenza vaccines that need to elicit broad and durable protection, such as the desired “universal” influenza vaccine, continued improvements to existing and alternative platforms are an important public health priority.
Data availability
All the data used to conceive and write this manuscript are in the public domain.
References
Garten, R. et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018-19 influenza vaccine. Morb. Mortal. Wkly. Rep. 67, 634–642 (2018).
Centers for Disease Control and Prevention. Weekly U.S. influenza surveillance report: FluView. https://www.cdc.gov/flu/weekly/index.htm (20 April 2018).
Schuchat, A. Transcript for CDC update on flu activity. https://www.cdc.gov/media/releases/2018/t0202-flu-update-activity.html (2 February 2018).
European Centre for Disease Prevention and Control. Weekly influenza update, week 15, April 2018. https://www.ecdc.europa.eu/en/publications-data/weekly-influenza-update-week-15-april-2018 (20 April 2018).
McCauley, J. M., Daniels, R. & Lin, Y. Report prepared for the WHO Annual Consultation on the Composition of Influenza Vaccine for the Northern Hemisphere 2018-2019 (WHO Collaborating Centre for Reference & Research on Influenza, London, UK, 2018).
Australian Influenza Surveillance Report No 10 – week ending 29 September 2017. (Australian Government, Department of Health, Canberra, 2017).
Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. https://www.cdc.gov/flu/about/disease/2015-16.htm (4 May 2018).
Vestergaard, L. S. et al. Excess all-cause and influenza-attributable mortality in Europe, December 2016 to February 2017. Euro. Surveill. 22(14):pii=30506 (2017).
Sullivan, S. G., Feng, S. & Cowling, B. J. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert. Rev. Vaccin. 13, 1571–1591 (2014).
Belongia, E. A. et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 16, 942–951 (2016).
Flannery, B. et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness - United States, February 2018. Morb. Mortal. Wkly. Rep. 67, 180–185 (2018).
Skowronski, D. M. et al. Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro. Surveill. 23(5):pii=18-00035 (2018).
Rondy, M. et al. Interim 2017/18 influenza seasonal vaccine effectiveness: combined results from five European studies. Euro. Surveill. 23(9 (21):pii=18-00086 (2018).
Sullivan, S. G. et al. Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Euro. Surveill. 22(43):pii=17-00707 (2017).
Flannery, B. et al. Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014-2015. J. Infect. Dis. 214, 1010–1019 (2016).
World Health Organization. Recommended composition of influenza virus vaccines for use in the 2018-2019 northern hemisphere influenza season. Wkly. Epidemiol. Rec. 93, 133–141 (2018).
Budd, A. P. et al. Update: influenza activity - United States, October 1, 2017-February 3, 2018. Morb. Mortal. Wkly. Rep. 67, 169–179 (2018).
Gottlieb, S. US House of Representatives, Testimony for the Committee on Energy and Commerce, Subcommittee on Oversight and Investigations: examining U.S. public health preparedness for and response efforts to seasonal influenza. Washington, DC. http://docs.house.gov/meetings/IF/IF02/20180308/106967/HHRG-115-IF02-Transcript-20180308.pdf (2018).
Skowronski, D. M. et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS ONE 9, e92153 (2014).
Robertson, J. S. Clinical influenza virus and the embryonated hen’s egg. Rev. Med. Virol. 3, 97–106 (1993).
Schild, G. C., Oxford, J. S., de Jong, J. C. & Webster, R. G. Evidence for host-cell selection of influenza virus antigenic variants. Nature 303, 706–709 (1983).
Katz, J. M. & Webster, R. G. Efficacy of inactivated influenza A virus (H3N2) vaccines grown in mammalian cells or embryonated eggs. J. Infect. Dis. 160, 191–198 (1989).
Rappuoli, R. et al. Public health. Rethink. Influenza Sci. 326, 50 (2009).
Hampson, A. et al. Improving the selection and development of influenza vaccine viruses - report of a WHO informal consultation on improving influenza vaccine virus selection, Hong Kong SAR, China, 18-20 November 2015. Vaccine 35, 1104–1109 (2017).
Frey, S. et al. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin. Infect. Dis. 51, 997–1004 (2010).
Seqirus. Seqirus Announces Next Major Advancement in Cell-Based Influenza Vaccine Technology(Holly Springs, NC, 2017.
Food and Drug Administration. Food and Drug Administration Letter of Approval: Biologics License Application Supplement for Influenza Vaccine (Flucelvax®) to include the use of MDCK cell isolated candidate vaccine virus strains obtained from CDC and the Victorian Infectious Diseases Reference Laboratory (Australia). (ed. CBER/FDA) Washington, D. C.: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM522280.pdf (2016).
Centers for Disease Control and Prevention. Cell-based flu vaccines. https://www.cdc.gov/flu/protect/vaccine/cell-based.htm(2018).
Lu, Y., Izurieta, H. S. & Forshee, R. Relative effectiveness of cell-cultured versus egg-based influenza vaccines, 2017–18. Advisory Committee on Immunization Practices (June 20, 2018) Y. Lu et al.; FDA/CBER (Centers for Disease Control and Prevention, Atlanta, 2018).
Lurie, N. HHS efforts to improve the influenza vaccine production enterprise. March 8, 2011, Briefing to The President’s Council of Advisors on Science and Technology: https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/pcast-lurie-march.pdf (2011).
Onions, D., Egan, W., Jarrett, R., Novicki, D. & Gregersen, J. P. Validation of the safety of MDCK cells as a substrate for the production of a cell-derived influenza vaccine. Biologicals 38, 544–551 (2010).
Minor, P. D. et al. Current challenges in implementing cell-derived influenza vaccines: implications for production and regulation, July 2007, NIBSC, Potters Bar, UK. Vaccine 27, 2907–2913 (2009).
Donis, R. O. et al. Performance characteristics of qualified cell lines for isolation and propagation of influenza viruses for vaccine manufacturing. Vaccine 32, 6583–6590 (2014).
Roth, B., Mohr, H., Enders, M., Garten, W. & Gregersen, J. P. Isolation of influenza viruses in MDCK 33016PF cells and clearance of contaminating respiratory viruses. Vaccine 30, 517–522 (2012).
Pyhala, R., Pyhala, L., Valle, M. & Aho, K. Egg-grown and tissue-culture-grown variants of influenza A (H3N2) virus with special attention to their use as antigens in seroepidemiology. Epidemiol. Infect. 99, 745–753 (1987).
Chen, Z., Zhou, H. & Jin, H. The impact of key amino acid substitutions in the hemagglutinin of influenza A (H3N2) viruses on vaccine production and antibody response. Vaccine 28, 4079–4085 (2010).
Saito, T. et al. Antigenic alteration of influenza B virus associated with loss of a glycosylation site due to host-cell adaptation. J. Med. Virol. 74, 336–343 (2004).
Furuya, Y. et al. Effect of inactivation method on the cross-protective immunity induced by whole ‘killed’ influenza A viruses and commercial vaccine preparations. J. Gen. Virol. 91, 1450–1460 (2010).
Kon, T. C. et al. Influenza vaccine manufacturing: effect of inactivation, splitting and site of manufacturing. Comparison of influenza vaccine production processes. PLoS ONE 11, e0150700 (2016).
She, Y. M., Cheng, K., Farnsworth, A., Li, X. & Cyr, T. D. Surface modifications of influenza proteins upon virus inactivation by beta-propiolactone. Proteomics 13, 3537–3547 (2013).
Dunkle, L. M. et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N. Engl. J. Med. 376, 2427–2436 (2017).
World Health Organization. Evaluation of Influenza Vaccine Effectiveness: A Guide to the Design and Interpretation of Observational Studies. (World Health Organization, Department of Immunization, Vaccines and Biologicals: Geneva, 2017.
Food and Drug Administration. Influenza Virus Vaccine for the 2017–2018 Season: Cumulative 2017/2018 Season Lot Release Status. Washington, DC. https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Post-MarketActivities/LotReleases/ucm567865.htm (2017).
Hughes, M. et al. Modeling projected impact of increased vaccination effectiveness and coverage on influenza burden - United States, 2016–2017 (ed. CDC) 67th Annual Epidemic Intelligence Service (EIS) Conference (CDC, Atlanta, GA, USA, 2018).
Vesikari, T. et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N. Engl. J. Med. 365, 1406–1416 (2011).
Izurieta, H. S. et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect. Dis. 15, 293–300 (2015).
Shay, D.K. et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J. Infect. Dis. 215, 510–517 (2017).
Landry, N. et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 5, e15559 (2010).
US. Public Law 109–417. 109th Congress: Pandemic and All-Hazards Preparedness Act. Washington, DC. https://www.gpo.gov/fdsys/pkg/PLAW-109publ417/pdf/PLAW-109publ417.pdf (2006).
Suzuki, Y., Odagiri, T., Tashiro, M. & Nobusawa, E. Development of an influenza A master virus for generating high-growth reassortants for A/Anhui/1/2013(H7N9) vaccine production in qualified MDCK cells. PLoS ONE 11, e0160040 (2016).
US Department of Health & Human Services. A milestone in protection from influenza. http://www.hhs.gov/news/press/2014pres/06/20140617a.html (2014).
Seqirus. Seqirus answers the call for better and faster influenza vaccine technologies. http://www.seqirus-us.com/media-room/press-releases/Seqirus-Answers-the-Call-for-Better-and-Faster-Influenza-Vaccine-Technologies (2018).
Protein Sciences Corporation. Superior Protection by Flublok® Influenza Vaccine in Seniors Documented in New England Journal of Medicine [press release, online]. http://www.proteinsciences.com/PDF/pscp2.pdf(2017).
Acknowledgements
We thank the members of the Influenza Cell Culture Vaccine Working Group, listed in the Appendix, and many others around the world who have directly contributed to the development of a new vaccine production system that utilizes viruses propagated in cell culture. We also thank WHO GISRS, Collaborating Centers, Essential Regulatory Laboratories, National Influenza Centers, vaccine manufacturers (IFPMA) for ongoing support of influenza vaccine development and evaluation. We thank Rick Bright and Julie Schafer for critical review of the manuscript. I.G.B. is employed by The Melbourne WHO Collaborating Centre for Reference and Research on Influenza, which is supported by the Australian Government Department of Health. R.O.D., D.E.W., and J.M.K. are employed by the US Department of Health and Human Services. J.W.M. is employed by the Crick Institute, an independent non-profit organization in the UK. T.O. is employed by National Institute of Infectious Diseases in Japan.
Author information
Authors and Affiliations
Contributions
I.G.B., R.O.D., J.M.K., J.W.M., T.O., H.T., T.F.T., and D.E.W. conceived, wrote, and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
WHO Collaborating Centers based in Melbourne (VIDRL) and Atlanta (Influenza Division, CDC) received funds under a Cooperative Research and Development Agreement with Seqirus to produce and characterize cell culture-based candidate vaccine viruses. I.G.B. owns shares in a company that produces influenza vaccine. T.F.T. is a full time employee of Takeda Vaccines, a manufacturer of cell culture-based pandemic influenza vaccines.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barr, I.G., Donis, R.O., Katz, J.M. et al. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: a step towards improved influenza vaccine effectiveness. npj Vaccines 3, 44 (2018). https://doi.org/10.1038/s41541-018-0079-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-018-0079-z
This article is cited by
-
CD46 inhibits the replication of swine influenza viruses by promoting the production of type I IFNs in PK-15 cells
Veterinary Research Communications (2024)
-
Redirecting antibody responses from egg-adapted epitopes following repeat vaccination with recombinant or cell culture-based versus egg-based influenza vaccines
Nature Communications (2024)
-
Targeting the hallmarks of aging to improve influenza vaccine responses in older adults
Immunity & Ageing (2023)
-
Enhanced isolation of influenza viruses in qualified cells improves the probability of well-matched vaccines
npj Vaccines (2021)
-
Influenza
Der Internist (2021)