Abstract
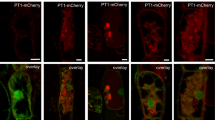
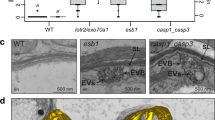
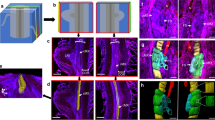
During establishment of arbuscular mycorrhizal symbioses, fungal hyphae invade root cells producing transient tree-like structures, the arbuscules, where exchange of photosynthates for soil minerals occurs. Arbuscule formation and collapse lead to rapid production and degradation of plant and fungal membranes, their spatiotemporal dynamics directly influencing nutrient exchange. We determined the ultra-structural details of both membrane surfaces and the interstitial apoplastic matrix by transmission electron microscopy tomography during growth and senescence of Rhizophagus irregularis arbuscules in rice. Invasive growth of arbuscular hyphae was associated with abundant fungal membrane tubules (memtubs) and plant peri-arbuscular membrane evaginations. Similarly, the phylogenetically distant arbuscular mycorrhizal fungus, Gigaspora rosea, and the fungal maize pathogen, Ustilago maydis, developed memtubs while invading host cells, revealing structural commonalities independent of the mutualistic or parasitic outcome of the interaction. Additionally, extracellular vesicles formed continuously in the peri-arbuscular interface from arbuscule biogenesis to senescence, suggesting an involvement in inter-organismic signal and nutrient exchange throughout the arbuscule lifespan.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the findings of this study are available from the corresponding author on request.
Change history
06 January 2021
In the version of this Article originally published, Supplementary Videos 1 and 2 were missing. These videos are now available.
References
Choi, J., Summers, W. & Paszkowski, U. Mechanisms underlying establishment of arbuscular mycorrhizal symbioses. Annu. Rev. Phytopathol. 56, 135–160 (2018).
Gutjahr, C. & Parniske, M. Cell biology: control of partner lifetime in a plant-fungus relationship. Curr. Biol. 27, R420–R423 (2017).
Balestrini, R., Hahn, M. G., Faccio, A., Mendgen, K. & Bonfante, P. Differential localization of carbohydrate epitopes in plant cell walls in the presence and absence of arbuscular mycorrhizal fungi. Plant Physiol. 111, 203–213 (1996).
Paszkowski, U., Kroken, S., Roux, C. & Briggs, S. P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl Acad. Sci. USA 99, 13324–13329 (2002).
Harrison, M. J., Dewbre, G. R. & Liu, J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14, 2413–2429 (2002).
Breuillin-Sessoms, F. et al. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 27, 1352–1366 (2015).
Kobae, Y. & Hata, S. Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plant Cell Physiol. 51, 341–353 (2010).
Javot, H., Pumplin, N. & Harrison, M. J. Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ. 30, 310–322 (2007).
Yang, S. Y. et al. Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the phosphate transporter1 gene family. Plant Cell 24, 4236–4251 (2012).
Roth, R. & Paszkowski, U. Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 39, 50–56 (2017).
Wewer, V., Brands, M. & Dormann, P. Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J. 79, 398–412 (2014).
Bravo, A., Brands, M., Wewer, V., Dormann, P. & Harrison, M. J. Arbuscular mycorrhiza-specific enzymes fatm and ram2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 214, 1631–1645 (2017).
Luginbuehl, L. H. et al. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356, 1175–1178 (2017).
Jiang, Y. et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356, 1172–1175 (2017).
Keymer, A. et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. eLife 6, e29107 (2017).
Keymer, A. & Gutjahr, C. Cross-kingdom lipid transfer in arbuscular mycorrhiza symbiosis and beyond. Curr. Opin. Plant Biol. 44, 137–144 (2018).
Zhang, Q., Blaylock, L. A. & Harrison, M. J. Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell 22, 1483–1497 (2010).
Kobae, Y. & Fujiwara, T. Earliest colonization events of Rhizophagus irregularis in rice roots occur preferentially in previously uncolonized cells. Plant Cell Physiol. 55, 1497–1510 (2014).
Girbardt, M. Über die Substruktur von Polystictus versicolor L.Arch. Mikrobiol. 28, 255–269 (1958).
Bonfantefasolo, P., Grippiolo, R. & Scannerini, S. Light and electron-microscopic features of the vesicular-arbuscular mycorrhiza in vitis roots. Caryologia 32, 120–120 (1979).
Gianinazzipearson, V. & Gianinazzi, S. Cellular and genetic aspects of interactions between hosts and fungal symbionts in mycorrhizae. Genome 31, 336–341 (1989).
Cox, G. & Sanders, F. Ultrastructure of host-fungus interface in a vesicular-arbuscular mycorrhiza. New Phytol. 73, 901–90 (1974).
Ivanov, S. et al. Nat. Plants https://doi.org/10.1038/s41477-019-0364-5 (2019).
Lanver, D. et al. The biotrophic development of Ustilago maydis studied by rna-seq analysis. Plant Cell 30, 300–323 (2018).
An, Q., Ehlers, K., Kogel, K. H., van Bel, A. J. & Huckelhoven, R. Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 172, 563–576 (2006).
An, Q., Huckelhoven, R., Kogel, K. H. & van Bel, A. J. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 8, 1009–1019 (2006).
Cai, Q. et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 360, 1126–1129 (2018).
Cocucci, E. & Meldolesi, J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 25, 364–372 (2015).
Barton, R. Electron microscope studies on surface activity in cells of chara vulgaris. Planta 66, 95–105 (1965).
Lucas, W. J., Keifer, D. W. & Pesacreta, T. C. Influence of culture-medium pH on charasome development and chloride transport in chara-corallina. Protoplasma 130, 5–11 (1986).
Moore, R. T. & Mcalear, J. H. Fine structure of mycota. 5. Lomasomes—previously uncharacterized hyphal structures. Mycologia 53, 194–19 (1961).
Marchant, R. & Moore, R. T. Lomasomes and plasmalemmasomes in fungi. Protoplasma 76, 235–247 (1973).
Pigott, C. D. Fine-structure of mycorrhiza formed by cenococcum-geophilum fr on tilia-cordata mill. New Phytol. 92, 501–512 (1982).
Heath, I. B. & Greenwoo, Ad. Structure and formation of lomasomes. J. Gen. Microbiol. 62, 129–12 (1970).
Bracker, C. E. Ultrastructure of fungi. Annu. Rev. Phytopathol. 5, 343–34 (1967).
Dexheimer, J., Gianinazzi, S. & Gianinazzipearson, V. Ultrastructural cytochemistry of the host-fungus interfaces in the endomycorrhizal association Glomus mosseae-Allium cepa . Pflanzenphysiol. 92, 191–206 (1979).
Scannerini, S. & Bonfantefasolo, P. Comparative ultrastructural analysis of mycorrhizal associations. Can. J. Bot. 61, 917–943 (1983).
Lefebvre, F. A. & Lecuyer, E. Small luggage for a long journey: transfer of vesicle-enclosed small RNA in interspecies communication. Front. Microbiol. 8, 377 (2017).
Marx, C., Dexheimer, J., Gianinazzipearson, V. & Gianinazzi, S. Enzymatic studies on the metabolism of vesicular-arbuscular mycorrhizas. 4. Ultracytoenzymological evidence (atpase) for active transfer processes in the host-arbuscule interface. New Phytol. 90, 37–43 (1982).
Gianinazzipearson, V., Smith, S. E., Gianinazzi, S. & Smith, F. A. Enzymatic studies on the metabolism of vesicular arbuscular mycorrhizas. 5. Is h+-atpase a component of atp-hydrolyzing enzyme-activities in plant fungus interfaces? New Phytol. 117, 61–74 (1991).
Gianinazzi-Pearson, V., Arnould, C., Oufattole, M., Arango, M. & Gianinazzi, S. Differential activation of H+-ATPase genes by an arbuscular mycorrhizal fungus in root cells of transgenic tobacco. Planta 211, 609–613 (2000).
Dexheimer, J., Marx, C., Gianinazzipearson, V. & Gianinazzi, S. Ultracytological studies of plasmalemma formations produced by host and fungus in vesiculararbuscular mycorrhizae. Cytologia 50, 461–471 (1985).
Micali, C. O., Neumann, U., Grunewald, D., Panstruga, R. & O’Connell, R. Biogenesis of a specialized plant-fungal interface during host cell internalization of Golovinomyces orontii haustoria. Cell Microbiol. 13, 210–226 (2011).
Rutter, B. D. & Innes, R. W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 173, 728–741 (2017).
Regente, M. et al. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 68, 5485–5495 (2017).
Helber, N. et al. A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp. is crucial for the symbiotic relationship with plants. Plant Cell 23, 3812–3823 (2011).
Djamei, A. et al. Metabolic priming by a secreted fungal effector. Nature 478, 395–398 (2011).
Lo Presti, L. et al. An assay for entry of secreted fungal effectors into plant cells. New Phytol. 213, 956–964 (2017).
McDonald, K. L. & Auer, M. High-pressure freezing, cellular tomography, and structural cell biology. Biotechniques 41, 137 (2006).
Hillmer, S., Viotti, C. & Robinson, D. G. An improved procedure for low-temperature embedding of high-pressure frozen and freeze-substituted plant tissues resulting in excellent structural preservation and contrast. J. Microsc. 247, 43–47 (2012).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Acknowledgements
We thank A. Bates and S. Gold for technical assistance, and Y. Kobae for kindly providing PT11-GFP transgenic rice lines. R. Roth was supported by Marie Curie FP7-PEOPLE-2013-IEF grant No. 629887 and by the Isaac Newton Trust RG74108; and U. Paszkowski by the BBSRC grant No. BB/N008723/1.
Author information
Authors and Affiliations
Contributions
R.R. and U.P. conceptualized the project. R.R., S.H. and C.F. carried out the experiments. R.R. and S.H. conducted the TEM and IGL analysis. C.F. and S.H. carried out the tomography and R.R. performed the IMOD 3D reconstruction. R.R. and M.C. did the quantitative analysis. R.R. and U.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal Peer Review Information Nature Plants thanks Rogers Innes, Erik Limpens and other anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7 and Supplementary Table 1.
Video 1
TEM tilt tomography and IMOD 3-D modeling shows memtubs, which remain connected with the fungal plasma membrane. Scale bar, 100 nm.
Video 2
TEM tilt tomography and IMOD 3-D modeling shows extracellular vesicles in the peri-arbuscular space and membrane evaginations continuous with the PAM. Scale bar, 100 nm.
Rights and permissions
About this article
Cite this article
Roth, R., Hillmer, S., Funaya, C. et al. Arbuscular cell invasion coincides with extracellular vesicles and membrane tubules. Nature Plants 5, 204–211 (2019). https://doi.org/10.1038/s41477-019-0365-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0365-4
This article is cited by
-
Extracellular RNAs released by plant-associated fungi: from fundamental mechanisms to biotechnological applications
Applied Microbiology and Biotechnology (2023)
-
Extracellular vesicles isolated from dsRNA-sprayed barley plants exhibit no growth inhibition or gene silencing in Fusarium graminearum
Fungal Biology and Biotechnology (2022)
-
Extracellular vesiculo-tubular structures associated with suberin deposition in plant cell walls
Nature Communications (2022)
-
Roles of RNA silencing in viral and non-viral plant immunity and in the crosstalk between disease resistance systems
Nature Reviews Molecular Cell Biology (2022)
-
In vitro resynthesis of lichenization reveals the genetic background of symbiosis-specific fungal-algal interaction in Usnea hakonensis
BMC Genomics (2020)