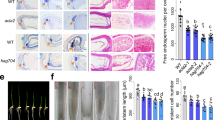
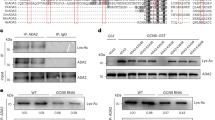
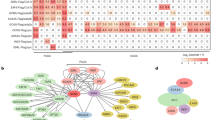
Abstract
Acetyl-coenzyme A (acetyl-CoA) is a central metabolite and the acetyl source for protein acetylation, particularly histone acetylation that promotes gene expression. However, the effect of acetyl-CoA levels on histone acetylation status in plants remains unknown. Here, we show that malfunctioned cytosolic acetyl-CoA carboxylase1 (ACC1) in Arabidopsis leads to elevated levels of acetyl-CoA and promotes histone hyperacetylation predominantly at lysine 27 of histone H3 (H3K27). The increase of H3K27 acetylation (H3K27ac) is dependent on adenosine triphosphate (ATP)-citrate lyase which cleaves citrate to acetyl-CoA in the cytoplasm, and requires histone acetyltransferase GCN5. A comprehensive analysis of the transcriptome and metabolome in combination with the genome-wide H3K27ac profiles of acc1 mutants demonstrate the dynamic changes in H3K27ac, gene transcripts and metabolites occurring in the cell by the increased levels of acetyl-CoA. This study suggests that H3K27ac is an important link between cytosolic acetyl-CoA level and gene expression in response to the dynamic metabolic environments in plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lu, C. & Thompson, C. B. Metabolic regulation of epigenetics. Cell Metab. 16, 9–17 (2012).
Verdin, E. & Ott, M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 16, 258–264 (2015).
Berr, A., Shafiq, S. & Shen, W. H. Histone modifications in transcriptional activation during plant development. Biochim Biophys Acta 1809, 567–576, (2011).
Kurdistani, S. K. & Grunstein, M. Histone acetylation and deacetylation in yeast. Nat. Rev. Mol. Cell Biol. 4, 276–284 (2003).
Cohen, H. Y. et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392 (2004).
Sack M. N., Finkel T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol. 4, a013102 (2012).
Takahashi, H., McCaffery, J. M., Irizarry, R. A. & Boeke, J. D. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol. Cell 23, 207–217 (2006).
Wellen, K. E., Hatzivassiliou, G., Sachdeva, U. M., Bui, T. V., Cross, J. R. & Thompson, C. B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Cai, L., Sutter, B. M., Li, B. & Tu, B. P. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell 42, 426–437 (2011).
Galdieri, L. & Vancura, A. Acetyl-CoA carboxylase regulates global histone acetylation. J. Biol. Chem. 287, 23865–23876 (2012).
Lee, J. V. et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 20, 306–319 (2014).
Madiraju, P., Pande, S. V., Prentki, M. & Madiraju, S. R. Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenetics 4, 399–403 (2009).
Sutendra, G. et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 158, 84–97 (2014).
Fatland, B. L. et al. Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol. 130,740–756 (2002).
Fatland, B. L., Nikolau, B. J. & Wurtele, E. S. Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis. Plant Cell 17, 182–203 (2005).
Oliver, D. J., Nikolau, B. J. & Syrkin Wurtele, E. Acetyl-CoA-Life at the metabolic nexus. Plant Sci. 176, 597–601 (2009).
Jin, H., Song, Z. & Nikolau, B. J. Reverse genetic characterization of two paralogous acetoacetyl CoA thiolase genes in Arabidopsis reveals their importance in plant growth and development. Plant J. 70, 1015–1032 (2012).
Yanai, Y., Kawasaki, T., Shimada, H., Wurtele, E. S., Nikolau, B. J. & Ichikawa, N. Genomic organization of 251 kDa acetyl-CoA carboxylase genes in Arabidopsis: tandem gene duplication has made two differentially expressed isozymes. Plant Cell Physiol. 36, 779–787 (1995).
Baud, S. et al. Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J. 33, 75–86 (2003).
Kajiwara, T., Furutani, M., Hibara, K. & Tasaka, M. The GURKE gene encoding an acetyl-CoA carboxylase is required for partitioning the embryo apex into three subregions in Arabidopsis. Plant Cell Physiol. 45, 1122–1128 (2004).
Tang, X. et al. The Arabidopsis BRAHMA chromatin-remodeling ATPase is involved in repression of seed maturation genes in leaves. Plant Physiol. 147, 1143–1157 (2008).
Lu, Q. et al. Arabidopsis homolog of the yeast TREX-2 mRNA export complex: components and anchoring nucleoporin. Plant J. 61, 259–270 (2010).
Tang, X. et al. Synergistic repression of the embryonic programme by SET DOMAIN GROUP 8 and EMBRYONIC FLOWER 2 in Arabidopsis seedlings. J. Exp. Bot. 63, 1391–1404 (2012).
Austin, R. S. et al. Next-generation mapping of Arabidopsis genes. Plant J. 67, 715–725 (2011).
Roudier, F. et al. Very-long-chain fatty acids are involved in polar auxin transport and developmental patterning in Arabidopsis. Plant Cell 22, 364–375 (2010).
Lu, S. et al. The glossyhead1 allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme A carboxylase in the biosynthesis of cuticular waxes by Arabidopsis. Plant Physiol. 157, 1079–1092 (2011).
Zuo, J., Niu, Q. W. & Chua, N. H. Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24, 265–273 (2000).
Charron, J. B., He, H., Elling, A. A. & Deng, X. W. Dynamic landscapes of four histone modifications during deetiolation in Arabidopsis. Plant Cell 21, 3732–3748 (2009).
Pandey, R. et al. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 30,5036–5055 (2002).
Biel, M., Kretsovali, A., Karatzali, E., Papamatheakis, J. & Giannis, A. Design, synthesis, and biological evaluation of a small-molecule inhibitor of the histone acetyltransferase Gcn5. Angew Chem. 43, 3974–3976 (2004).
Malapeira, J., Khaitova, L. C. & Mas, P. Ordered changes in histone modifications at the core of the Arabidopsis circadian clock. Proc. Natl Acad. Sci. USA 109, 21540–21545 (2012).
Earley, K. W., Shook, M. S., Brower-Toland, B., Hicks, L. & Pikaard, C. S. In vitro specificities of Arabidopsis co-activator histone acetyltransferases: implications for histone hyperacetylation in gene activation. Plant J. 52, 615–626 (2007).
Xiao, J. et al. Requirement of histone acetyltransferases HAM1 and HAM2 for epigenetic modification of FLC in regulating flowering in Arabidopsis. Plant Physiol. 170, 444–451 (2013).
Bordoli, L., Netsch, M., Luthi, U., Lutz, W. & Eckner, R. Plant orthologs of p300/CBP: conservation of a core domain in metazoan p300/CBP acetyltransferase-related proteins. Nucleic Acids Res. 29, 589–597 (2001).
Long, J. A., Ohno, C., Smith, Z. R. & Meyerowitz, E. M. TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312, 1520–1523 (2006).
Berna, G., Robles, P. & Micol, J. L. A mutational analysis of leaf morphogenesis in Arabidopsis thaliana. Genetics 152, 729–742 (1999).
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R. & Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78, 779–787 (2006).
Shyh-Chang, N. et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339, 222–226 (2013).
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F. & Kroemer, G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21,805–821 (2015).
Shen, Y., Wei, W. & Zhou, D.-X. Histone acetylation enzymes coordinate metabolism and gene expression. Trends Plant Sci. 20, 614–621 (2015).
Chow, J. D. et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol. Metab. 3, 419–431 (2014).
Kim, J. M. et al. Acetate-mediated novel survival strategy against drought in plants. Nat. Plants 3, 17097 (2017).
Benhamed, M., Bertrand, C., Servet, C. & Zhou, D. X. Arabidopsis GCN5, HD1, and TAF1/HAF2 interact to regulate histone acetylation required for light-responsive gene expression. Plant Cell 18, 2893–2903 (2006).
McMillan, A., Renaud, J. B., Gloor, G. B., Reid, G. & Sumarah, M. W. Post-acquisition filtering of salt cluster artefacts for LC-MS based human metabolomic studies. J. Cheminform. 8, 44 (2016).
Servet, C., Conde e Silva, N. & Zhou, D. X. Histone acetyltransferase AtGCN5/HAG1 is a versatile regulator of developmental and inducible gene expression in Arabidopsis. Mol. Plant 3, 670–677 (2010).
Henry, R. A., Kuo, Y. M. & Andrews, A. J. Differences in specificity and selectivity between CBP and p300 acetylation of histone H3 and H3/H4. Biochemistry 52, 5746–5759 (2013).
Henry, R. A., Kuo, Y. M., Bhattacharjee, V., Yen, T. J. & Andrews, A. J. Changing the selectivity of p300 by acetyl-CoA modulation of histone acetylation. ACS Chem. Biol. 10, 146–156 (2015).
Curtis, M. D. & Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003).
Schwab, R., Ossowski, S., Riester, M., Warthmann, N. & Weigel, D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18, 1121–1133 (2006).
Adachi, S., Nobusawa, T. & Umeda, M. Quantitative and cell type-specific transcriptional regulation of A-type cyclin-dependent kinase in Arabidopsis thaliana. Dev. Biol. 329, 306–314 (2009).
Tsuchiya, Y., Pham, U., Hu, W., Ohnuma, S. & Gout, I. Changes in acetyl CoA levels during the early embryonic development of Xenopus laevis. PLoS One 9, e97693 (2014).
Li, C. et al. Concerted genomic targeting of H3K27 demethylase REF6 and chromatin-remodeling ATPase BRM in Arabidopsis. Nat. Genet. 48, 687–693 (2016).
Kim, D., Pertea, G., Trapnell, C., Pimentel, H., Kelley, R. & Salzberg, S. L. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Gendrel, A. V., Lippman, Z., Martienssen, R. & Colot, V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2, 213–218 (2005).
Li, C. et al. The Arabidopsis SWI2/SNF2 chromatin remodeler BRAHMA regulates polycomb function during vegetative development and directly activates the flowering repressor gene SVP. PLoS Genet. 11, e1004944 (2015).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucl. Acids Res. 44, W160–165 (2016).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Nicol, J. W., Helt, G. A., Blanchard, S. G. Jr., Raja, A. & Loraine, A. E. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics 25, 2730–2731 (2009).
Zang, C., Schones, D. E., Zeng, C., Cui, K., Zhao, K. & Peng, W. A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics 25, 1952–1958 (2009).
Salmon-Divon, M., Dvinge, H., Tammoja, K. & Bertone, P. PeakAnalyzer: genome-wide annotation of chromatin binding and modification loci. BMC Bioinformatics 11, 415 (2010).
Zheng, Q. & Wang, X. J. GOEAST: a web-based software toolkit for gene ontology enrichment analysis. Nucleic Acids Res. 36, W358–363 (2008).
Kessner, D., Chambers, M., Burke, R., Agus, D. & Mallick, P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics 24,2534–2536 (2008).
Tautenhahn, R., Bottcher, C. & Neumann, S. Highly sensitive feature detection for high resolution LC/MS. BMC Bioinform. 9, 504 (2008).
Prince, J. T. & Marcotte, E. M. Chromatographic alignment of ESI-LC-MS proteomics data sets by ordered bijective interpolated warping. Anal. Chem. 78,6140–6152 (2006).
Acknowledgements
We thank R. Menassa and N. Hüner for helpful discussions, M. Tasaka for the seeds of gk101 (acc1-4), J.L. Micol for the seeds of elo3 (elp3), ABRC for the seeds of T-DNA insertion lines, and A. Molnar for help with preparing the figures. This work was supported by grants from the Natural Science and Engineering Research Council of Canada (R4019A01, to Y.C.), Agriculture and Agri-Food Canada (to Y.C.) and National Natural Science Foundation of China (31371715, to J.L.). C.C. was supported by a graduate fellowship from the Chinese Scholarship Council.
Author information
Authors and Affiliations
Contributions
C.C. and Y.C. conceived and designed the experiments; C.C., Y.W. and A.H. quantified the level of acetyl-CoA; J.R and C.C performed metabolome profiling; J.R., F. M. and S.K. analysed the metablomic data; G.T., B.S. and R.S.A. conducted mapping of p1045. V.N. and Z.C.Y. performed all the Sanger DNA sequencing; C.C. performed all the rest of the experiments; C.C., C.L., Z.C.Y., J.L., K.Y., S.E.K., S.H. and K.W. analysed ChIP-seq and RNA-seq data; C.C., C.L. and Y.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Figures 1-18, Supplementary Tables 1-3
41477_2017_23_MOESM5_ESM.xlsx
Supplementary Dataset 3 List of genes with increased and decreased acetylations (at lysine residues other than H3K27) in acc1-5
41477_2017_23_MOESM6_ESM.xlsx
Supplementary Dataset 4 Transcripts significantly changed in acc1-5 identified from RNA-seq and lists of genes in 7 functional categories
Rights and permissions
About this article
Cite this article
Chen, C., Li, C., Wang, Y. et al. Cytosolic acetyl-CoA promotes histone acetylation predominantly at H3K27 in Arabidopsis . Nature Plants 3, 814–824 (2017). https://doi.org/10.1038/s41477-017-0023-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-017-0023-7
This article is cited by
-
Lysine acetylation of histone acetyltransferase adaptor protein ADA2 is a mechanism of metabolic control of chromatin modification in plants
Nature Plants (2024)
-
Interplay between coding and non-coding regulation drives the Arabidopsis seed-to-seedling transition
Nature Communications (2024)
-
The tRNA-degradation pathway impacts the phenotype and metabolome of Arabidopsis thaliana: evidence from atipt2 and atipt9 knockout mutants
Plant Growth Regulation (2024)
-
DDM1-mediated gene body DNA methylation is associated with inducible activation of defense-related genes in Arabidopsis
Genome Biology (2023)
-
ACL and HAT1 form a nuclear module to acetylate histone H4K5 and promote cell proliferation
Nature Communications (2023)