Abstract
Biarylphosphines have been widely applied as ligands in various synthetic methods, especially in transition-metal-catalysed carbon-carbon and carbon-heteroatom bond cross-coupling reactions. Based on the outstanding properties of the parent scaffolds, a general method for in situ modification of the commercial tertiary phosphine ligands to access a series of ligands is in high demand. Here we show that a rhodium-catalysed system is introduced for the hydroarylation of alkenes and alkynes with tertiary phosphines through P(III)-chelation assisted C-H activation. A series of ligand libraries containing alkyl and alkenyl substituted groups with different steric and electronic properties are obtained in high yields. Furthermore, several experimental studies are performed to uncover the key mechanistic features of the linear-selective hydroarylation of alkenes and branch-selective hydroarylation of alkynes.
Similar content being viewed by others
Introduction
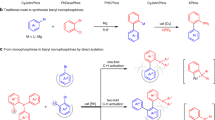
Transition-metal-catalysed C−H activation assisted by chelating directing groups is a well-established strategy1,2,3,4,5,6,7,8,9,10. Of these processes, the hydroarylation of C−C double and triple bonds through C−H addition has received much attention recently because it represents a versatile and atom-economical method11,12,13,14. A remarkable feature of chelation-assisted hydroarylation resides in the ability to overcome the inert nature of C−H bonds and control site-selectivity in molecules with diverse C−H bonds. In 1993, Murai et al.15 reported a significant, pioneering work on the Ru-catalysed hydroarylation of alkenes with aromatic ketones by carbonyl-directed C−H activation. Since then, the emergence of promising O-chelation assisted hydroarylations in various substrates such as N,N-dimethylcarbamoyl indoles16, N,N-diethylbenzamides17,18, anilides19, enamides20,21,22,23 and so on by rhodium and iridium catalysts has been reported (Fig. 1a)24. In the ensuing years, the catalytic hydroarylation reactions have focused on the development of N directing groups with different transition metal catalysts (Fig. 1b)25,26,27,28,29,30,31,32,33,34. Since 2010, Yoshikai and co-workers35,36,37,38,39,40 have reported a series of cobalt-catalysed hydroarylation of alkenes and alkynes with 2-phenylpyridines and imines. In 2013, Wang and Chen uncovered the Mn(I)-catalysed aromatic C−H alkenylation of terminal alkynes with 2-phenylpyridines41,42. In 2018, Ackermann and co-workers43 reported the enantioselective cobalt(III)-catalysed C-H alkylation of indole at C2 position with alkenes by an N-pyridine-type directing group. A very recent example by Yu, Sun and Lu involved a U-shaped nitrile template-directed, Rh(III)-catalysed meta-C−H alkenylation of arenes with disubstituted alkynes, wherein the chelation of cyano group was crucial for the meta-selective hydroarylation44. Despite these widely acknowledged advances, a variation to the original Murai protocol, which enables catalytic P-chelated, regioselective hydroarylation remains elusive.
Towards a catalytic process for hydroarylation of alkenes and alkynes with diarylphosphines through P(III)-chelation-assisted C−H activation. a O-Chelation-assisted hydroarylation. b N-Chelation-assisted hydroarylation. c Precedent studies on P-chelated transition metal-olefin complexes. d Transition-metal-catalysed C–H borylation and arylation of diarylphosphines. e Rh-catalysed P(III)-chelation-assisted hydroarylation of alkenes and alkynes with diarylphosphines
P-chelated transition metal-olefin complexes45 with an aromatic C−H bond have been studied from a stoichiometric point of view. In 2011, Schauer and Brookhart uncovered the migratory insertion of square-pyramidal d6, 16-electron Ir(III), and Rh(III) olefin hydride complexes assisted by a (bis)chelated O-P(III) motif (Fig. 1c)46. Due to the high stability of pincer complexes47,48, subsequent reductive elimination to build the C(sp2)–C(sp3) bond could not proceed. Furthermore, the catalytic variation is even more challenging. Recently, we49 and Clark50,51 have disclosed C–H arylation and borylation of biaryl phosphines, in which the inherent monodentate phosphine was used as a directing group (Fig. 1d). The development of the PIII-chelation-assisted C–H activation in a catalytic process prompted us to consider whether these phosphines could directly undergo hydroarylation by transition metal catalysts52,53,54,55,56,57,58. Inspired by these previous results, herein, we report a catalytic transformation for the regioselective hydroarylation of commercially available tertiary phosphines with alkenes and alkynes through a rhodium-catalysed, P(III)-directed C–H alkylation and alkenylation (Fig. 1e). Such routes are particularly desirable because alkyl and alkenyl-substituted phosphine ligands59,60,61 with different steric and electronic properties can be produced by an in situ modification strategy62.
Results
Reaction design
We initiated our study by investigating the reaction of phosphine 1a with methyl acrylate (2a) in the presence of an array of rhodium and iridium catalysts (Table 1). As a result, we discovered that the use of 2.5 mol% [Rh(cod)Cl]2 and 3.0 equivalents of NaHCO3 in toluene at 140 °C for 3 h led to the formation of alkylation product 3aa with a 75% yield and a small amount of disubstituted alkylation product 3aa′ (Table 1, entry 1). Other rhodium complexes such as [Rh(coe)2Cl]2 (Table 1, entry 2) and [Rh(CO)2Cl]2 (Table 1, entry 3) were less efficient for this reaction. When the reaction was carried out using iridium catalysts such as [Ir(cod)Cl]2, we did not observe any C–H alkylation products (Table 1, entry 4). Further control experiments confirmed that the coupling process did not occur in the absence of the Rh catalyst (Table 1, entry 5) and the conversion was poor without NaHCO3 (Table 1, entry 6). Other bases such as Na2CO3 led to dramatic erosions in yield (Table 1, entry 7). The non-polar solvent was required for the reaction and the use of xylene as a solvent led to a slightly lower yield (Table 1, entry 8). Lowering the temperature to 130 °C had a little impact on the reaction outcome (Table 1, entry 9); however, at a lower temperature, 100 °C the result was found much inferior than that observed under the optimal conditions (Table 1, entry 10).
Scope of the methodology
The scope of the mono-substituted alkylation products were examined using a wide range of phosphine ligands. As shown in Fig. 2, biaryl phosphines bearing Me (1b–1c), OMe (1d), NMe2 (1e) and F (1f) substituents underwent facile hydroarylation with methyl acrylate (2a), affording the corresponding products 3ba–3fa with 53–83% yields. Substrate 1g, containing a naphthalen-2-yl group, was also compatible, and the C–H activation was located at the less sterically hindered C3 position. CyJohnPhos (1h), 2-(dicyclohexylphosphino)-2′-methoxybiphenyl (1i), and Davephos (1j) with a PCy2 directing group produced hydroarylation products 3ha–3ja with 47–67% yields. Furthermore, JohnPhos (1k), having a sterically hindered PtBu2 group, produced the desired product 3ka in modest yield. The cataCxium ligand series, developed by Beller, such as cataCXium® PPh (1l) and cataCXium® PCy (1m), were compatible as well. Under the optimized conditions, phosphine 1a was effectively coupled to olefins such as butyl acrylate (2b), (vinylsulfonyl)benzene (2d), and styrenes 2d–e in high efficiency and regioselectivity. Reactions conducted with acrylonitrile (2f) formed a linear hydroarylation product 3af with 80% yield, which was confirmed by X-ray analysis. Notably, internal olefins such as methyl (E)-but-2-enoate (2g) were tolerated as well.
Rhodium-catalysed direct hydroarylation of alkenes with phosphines. Reaction conditions: 2.5 mol% [Rh(cod)Cl]2, 1 (0.20 mmol), 2 (0.20 mmol), NaHCO3 (0.60 mmol) in 1 mL toluene, at 140 °C, 3–4 h, under argon. All reported yields are isolated yields. aUsing 2 (0.60 mmol) at 150 °C for 24 h. b5 mol% [Rh(coe)2Cl]2, 1 (0.20 mmol), 2 (0.60 mmol), K2CO3 (0.60 mmol) in 1 mL toluene, at 150 °C, 24 h, under argon. cUsing 5 mol% [Rh(cod)Cl]2, 2 (0.60 mmol) at 150 °C for 24 h
Under slightly modified conditions, the reaction of phosphine 1a (1.0 equiv) and 1-ethynyl-4-methoxybenzene (4a, 2.0 equiv) in the presence of 2.5 mol% [Rh(cod)Cl]2 without NaHCO3, at 120 °C under an Ar atmosphere in toluene, formed a Markovnikov hydroarylation adduct 5aa in 85% yield (Fig. 3). Then we studied the scope of the hydroarylation reaction between phosphine ligands and alkynes for the
synthesis of alkenyl-substituted products. Unlike the above reactivity, this olefination reaction is sensitive to the steric hindrance of the phosphines. Therefore, the C2-substituted phosphines 1b, 1d–e and 1i–j and the sterically hindered JohnPhos (1k) with the PtBu2 directing group were not tolerated. Phosphines such as 1c, 1f–h and 1l–m were applied in this C–H olefination reaction. Terminal alkynes, including phenylacetylene (4b), as well as derivatives that incorporate methyl (4c), phenyl (4d) and Cl (4e) on arenes, which were readily tolerated (5ab–5ae). As an example of a heteroaromatic alkyne, 3-ethynylthiophene (4f) was shown to be amenable to this protocol. Furthermore, the hydroarylation of alkyl-substituted terminal alkynes 4g–i proceeded smoothly with branch-selectivity.
In addition to the mono-selective hydroarylation, we also investigated the disubstituted products. To increase the di-selectivity to a more synthetically useful level, phosphine 1a was treated with 3.0 equiv of 2a and 3.0 equiv of NaHCO3 in toluene at 150 °C for 24 h to selectively form the disubstituted product 3aa′ with a 78% yield, which was confirmed by X-ray analysis (Fig. 4a). Other olefins such as butyl acrylate (2b) and other phosphines such as cataCXium® PCy (1l) produced the corresponding disubstituted hydroarylation products 3ab′ and 3ka′ in good yields. However, the C–H alkenylation reactions only displayed mono-selectivity. For instance, the reaction of phosphine 1a with a large amount of alkyne 4a still generated the mono-substituted product 5aa and the corresponding disubstituted product 5aa′ was not detected. Besides the Markovnikov selectivity observed in Fig. 3, interestingly, an extremely bulky TIPS substituted terminal alkyne 6 only produced the product 7 in an anti-Markovnikov mode (Fig. 4b).
Synthetic applications
Besides diarylphosphines, this strategy can also be extended to binaphthyl-based chiral phosphine ligands. Notably, alkyl, ether and dialkylamino groups are widely used to adjust the steric structure in these phosphines. Based on this method, the alkyl groups could be rapidly and efficiently installed by P(III)-directed C–H alkylation of alkenes. For example, when (R)-H-MOP (8) was employed with olefin 2 h, the alkylation product 9 was generated in 56% yield without erosion of ee (Fig. 5a). To further modulate the steric properties of the substituent at alkyl substituents, olefins 2i and 2j with bulky substituents were employed, providing the compounds 10–11 with acceptable yields and excellent stereochemical reliability. To show the synthetic utility, we next tested them as ligands in transition metal-catalysed asymmetric reactions. As shown in Fig. 5b, following the procedures disclosed by the Hayashi research group on the Rh-catalysed arylation of related isatins 12a–b with PhB(OH)2 (13) using the best ligand (R)-MeO-MOP, alcohols 14a–b were obtained in good yields with 75%63 and 89% ee64, respectively. Furthermore, when the modified ligands 9–11 were screened, the enantioselectivity of the product 12 could be dramatically improved to 84% ee. In addition, compound 9 showed a better reactivity for the synthesis of the product 12b.
Discussion
To probe the reaction mechanism, we conducted a series of experimental investigations (Fig. 6). Reactions of the [Rh(cod)Cl]2 dimer with phosphine 1a (δP = −13.6 ppm) in toluene at room temperature generated a yellow complex 15 (δP = 20.5 ppm), as confirmed by X-ray analysis and 31P NMR spectroscopy. Detected by the 31P NMR spectrum, no new signal appeared when the solution 6 was heated in D8-toluene at 120 °C65,66. Addition of alkene 2a to the above system generated two new signals, confirmed as intermediate 16 (δP = 36.7 ppm) and the product 3aa′ (δP = −15.0 ppm). Following a similar protocol for alkyne 4a resulted in the formation of compounds 17 (δP = 35.7 ppm) and 5aa (δP = −14.9 ppm). Further detected by high-resolution mass spectrometry (HRMS), the analysed samples 16 and 17 showed diminished signals for the precursors of products 3aa′ and 5aa. This result indicates that the C–H activation event is triggered by the addition of the alkene or alkyne, and phosphine chelation with the rhodium species is crucial for this regio-controlled hydroarylation.
To gain further insight into the reaction mechanism, deuterated experiments were carried out by D-1a with 2a in the standard reaction conditions. 1H NMR analysis revealed that D/H-exchange was detected on the D5-benzonic core of 1a, and 42% and 3% deuterium incorporation at the β and α positions, respectively, were observed after 3 h. Moreover, 50% deuterium incorporation was detected at the β-olefinic position of D-5aa, without stereoselectivity (Fig. 7a). In addition, the KIE values of the C–H activation in hydroarylation of 2a and 4a with phosphine 1a were 2.5 and 3.5, respectively, revealing that the C–H cleavage is slow, and involved as a rate-determining step in both alkylation and alkenylation process (Fig. 7b).
Based upon the results herein and the precedent reports, plausible reaction pathways are shown in Fig. 8. The rhodium species RhX first coordinates to the P atom of phosphine D5–1, which leads to the formation of complex A. A reversible coordination with alkene 2 or alkyne 4 delivers the intermediate B45. In the case of methyl acrylate (2a), subsequent C–H activation takes place and delivers the alkene-hydride complex C. The deuterium labelling experiments demonstrates the cis insertion of the Rh–D bond into 2a, which first occurs at the α-position to generate intermediate D46, then undergoes reversible β-hydride elimination to form the olefin complex E67 and eventually produces the β-addition complex F. In the alkyne 4a system, the Rh–alkyne complex B undergoes C–H activation to form rhodacycle C′, which undergoes a hydride addition at the α-position to generate intermediate D′. If reductive elimination happens at this step, we should only observe the trans-hydroarylation product D-5aa. The nonstereospecific deuteration indicates that the species F′ is possibly generated from carbenoid E′68 via isomerization of the D′ species. Then, intermediates F and F′ produce complex G via reductive elimination, which generates the desired products 3 and 5 and then reforms catalytic species A via exchange with another phosphine molecule.
In summary, we developed an effective rhodium-catalysed system that can activate the aromatic C–H bonds of phosphines with alkenes and alkynes through a hydroarylation process. A diverse class of alkyl and phosphine ligands with different steric and electronic properties are obtained in moderate to good yields with excellent site-selectivity. Additional applications of the developed ligand libraries and more detailed mechanistic studies are also ongoing in our laboratory.
Methods
General procedures for synthesis of 3
In an oven-dried Schlenk tube, 1 (1.0 equiv, 0.20 mmol), 2 (1.0 equiv, 0.20 mmol), [Rh(cod)Cl]2 (2.5 mol%, 2.5 mg, 0.005 mmol), NaHCO3(3.0 equiv, 50.4 mg, 0.60 mmol) were dissolved in freshly distilled toluene (1.0 mL). The mixture was stirred at 140 °C under argon for 3 h. Upon the completion of the reaction, the solvent was removed. The crude mixture was directly subjected to column chromatography on silica gel using petrol ether/EtOAc as eluent to give the desired products.
General procedures for synthesis of 5
In an oven-dried Schlenk tube, 1 (1.0 equiv, 0.20 mmol), 4 (2.0 equiv, 0.40 mmol), [Rh(cod)Cl]2 (2.5 mol%, 2.5 mg, 0.005 mmol) were dissolved in freshly distilled toluene (1.0 mL). The mixture was stirred at 120 °C under argon for 12 h. Upon the completion of the reaction, the solvent was removed. The crude mixture was directly subjected to column chromatography on silica gel using petrol ether/EtOAc as eluent to give the desired products.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1893756, CCDC 1893758, CCDC 1893759 and CCDC 1893762. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Lyons, T. W. & Sanford, M. S. Palladium-catalysed ligand-directed C−H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Engle, K. M., Mei, T.-S., Wasa, M. & Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C−H functionalization reaction. Acc. Chem. Res. 45, 788–802 (2012).
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C−H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Colby, D. A., Tsai, A. S., Bergman, R. G. & Ellman, J. A. Rhodium catalysed chelation-assisted C−H bond functionalization reactions. Acc. Chem. Res. 45, 814–825 (2012).
Arockiam, P. B., Bruneau, C. & Dixneuf, P. H. Ruthenium(II)-catalysed C–H bond activation and functionalization. Chem. Rev. 112, 5879 (2012).
Zhang, F. & Spring, D. R. Arene C–H functionalisation using a removable/modifiable or a traceless directing group strategy. Chem. Soc. Rev. 43, 6906–6919 (2014).
Gensch, T., Hopkinson, M. N., Glorius, F. & Wencel-Delord, J. Mild metal-catalysed C–H activation: examples and concepts. Chem. Soc. Rev. 45, 2900–2936 (2016).
He, J. et al. Palladium-catalysed alkyl C-H bond activation. Chem. Rev. 117, 8754 (2017).
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Gandeepan, P. et al. Transition metals for C–H activation. Chem. Rev https://doi.org/10.1021/acs.chemrev.8b00507.
Gao, K. & Yoshikai, N. Low-valent cobalt catalysis: new opportunities for C–H functionalization. Acc. Chem. Res. 47, 1208–1219 (2014).
Yamamoto, Y. Synthesis of heterocycles via transition-metal-catalysed hydroarylation of alkynes. Chem. Soc. Rev. 43, 1575–1610 (2014).
Boyarskiy, V. P., Ryabukhin, D. S., Bokach, N. A. & Vasilyev, A. V. Alkenylation of arenes and heteroarenes with alkynes. Chem. Rev. 116, 5894–5986 (2016).
Dong, Z. et al. Transition-metal-catalysed C−H alkylation using alkenes. Chem. Rev. 117, 9333–9403 (2017).
Murai, S. et al. Efficient catalytic addition of aromatic carbon-hydrogen bonds to olefins. Nature 366, 529–531 (1993).
Schipper, D. J., Hutchinson, M. & Fagnou, K. Rhodium(III)-catalysed intermolecular hydroarylation of alkynes. J. Am. Chem. Soc. 132, 6910–6911 (2010).
Crisenza, G. E. M., McCreanor, N. G. & Bower, J. F. Branch-selective, iridium-catalysed hydroarylation of monosubstituted alkenes via a cooperative destabilization strategy. J. Am. Chem. Soc. 136, 10258–10259 (2014).
Huang, G. & Liu, P. Mechanism and origins of ligand-controlled linear versus branched selectivity of iridium-catalysed hydroarylation of alkenes. ACS Catal. 6, 809–820 (2016).
Grélaud, S., Cooper, P., Feron, L. J. & Bower, J. F. Branch-selective and enantioselective iridium-catalysed alkene hydroarylation via anilide-directed C–H oxidative addition. J. Am. Chem. Soc. 140, 9351–9356 (2018).
Mo, F. & Dong, G. Regioselective ketone α-alkylation with simple olefins via dual activation. Science 345, 68–72 (2014).
Mo, F., Lim, H. N. & Dong, G. Bi-functional ligand-assisted catalytic ketone α-alkenylation with internal alkynes: controlled synthesis of enones and mechanistic studies. J. Am. Chem. Soc. 137, 15518–15527 (2015).
Xing, D. & Dong, G. Branched-selective intermolecular ketone α-alkylation with unactivated alkenes via an enamide directing strategy. J. Am. Chem. Soc. 139, 13664–13667 (2017).
Xing, D. et al. Branched-selective direct α-alkylation of cyclic ketones with simple alkenes. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.201900301 (2019).
Huang, Z. et al. Transition metal-catalysed ketone-directed or mediated C–H functionalization. Chem. Soc. Rev. 44, 7764–7786 (2015).
Lim, S.-G., Lee, J. H., Moon, C. W., Hong, J.-B. & Jun, C.-H. Rh(I)-catalysed direct ortho-alkenylation of aromatic ketimines with alkynes and its application to the synthesis of isoquinoline derivatives. Org. Lett. 5, 2759–2761 (2003).
Cheng, K., Yao, B., Zhao, J. & Zhang, Y. RuCl3-catalysed alkenylation of aromatic C−H bonds with terminal alkynes. Org. Lett. 10, 5309–5312 (2008).
Katagiri, T., Mukai, T., Satoh, T., Hirano, K. & Miura, M. Rhodium-catalysed and coordination-assisted regioselective alkenylation of aromatic C–H bonds with terminal silylacetylenes. Chem. Lett. 38, 118–119 (2009).
Kwak, J., Ohk, Y., Jung, Y. & Chang, S. Rollover cyclometalation pathway in rhodium catalysis: dramatic NHC effects in the C−H bond functionalization. J. Am. Chem. Soc. 134, 17778–17788 (2012).
Ebe, Y. & Nishimura, T. Iridium-catalysed branch-selective hydroarylation of vinyl ethers via C–H bond activation. J. Am. Chem. Soc. 137, 5899–5902 (2015).
Shirai, T. & Yamamoto, Y. Cationic iridium/S‐Me-BIPAM-catalysed direct asymmetric intermolecular hydroarylation of bicycloalkene. Angew. Chem. Int. Ed. 54, 9894–9897 (2015).
Zhou, X. et al. Cp*CoIII-catalysed branch-selective hydroarylation of alkynes via C–H activation: efficient access to α-gem-vinylindoles. ACS Catal. 7, 7296–7304 (2017).
Zell, D. et al. Full selectivity control in cobalt(III)-catalysed C−H alkylations by switching of the C−H activation mechanism. Angew. Chem. Int. Ed. 56, 10378–10382 (2017).
Ebe, Y., Onoda, M., Nishimura, T. & Yorimitsu, H. Iridium-catalysed regio- and enantioselective hydroarylation of alkenyl ethers by olefin isomerization. Angew. Chem. Int. Ed. 56, 5607–5611 (2017).
Lorion, D. M. et al. Late‐stage peptide diversification through cobalt-catalysed C−H activation: sequential multicatalysis for stapled peptides. Angew. Chem. Int. Ed. 58, 1684–1688 (2019).
Gao, Ke, Lee, P.-S., Fujita, T. & Yoshikai, N. Cobalt-catalysed hydroarylation of alkynes through chelation-assisted C−H bond activation. J. Am. Chem. Soc. 132, 12249–12251 (2010).
Lee, P.-S., Fujita, T. & Yoshikai, N. Cobalt-catalysed, room-temperature addition of aromatic imines to alkynes via directed CH bond activation. J. Am. Chem. Soc. 133, 17283–17295 (2011).
Gao, Ke. & Yoshikai, N. Regioselectivity-switchable hydroarylation of styrenes. J. Am. Chem. Soc. 133, 400–402 (2011).
Xu, W. & Yoshikai, N. Highly linear selective cobalt-catalysed addition of aryl imines to styrenes: reversing intrinsic regioselectivity by ligand elaboration. Angew. Chem. Int. Ed. 53, 14166–14394 (2014).
Lee, P. S. & Yoshikai, N. Aldimine-directed branched-selective hydroarylation of styrenes. Angew. Chem. Int. Ed. 52, 1240–1244 (2013).
Xu, W. & Yoshikai, N. N−H imine as a powerful directing group for cobalt-catalysed olefin hydroarylation. Angew. Chem. Int. Ed. 55, 12731–12735 (2016).
Zhou, B., Chen, H. & Wang, C. Mn-catalysed aromatic C−H alkenylation with terminal alkynes. J. Am. Chem. Soc. 135, 1264–1267 (2013).
Hammarback, L. A., Robinson, A., Lynam, J. M. & Fairlamb, I. J. S. Mechanistic insight into catalytic redox-neutral C−H bond activation involving manganese(I) carbonyls: catalyst activation, turnover, and deactivation pathways reveal an intricate network of steps. J. Am. Chem. Soc. 141, 2316–2328 (2019).
Pesciaioli, F. et al. Enantioselective cobalt(III)-catalysed C-H activation enabled by chiral carboxylic acid cooperation. Angew. Chem. Int. Ed. 57, 15425–15429 (2018).
Xu, H.-J. et al. Rh(III)-catalysed meta-C-H alkenylation with alkynes. J. Am. Chem. Soc. 141, 76–79 (2019).
Johnson, J. B. & Rovis, T. More than bystanders: the effect of olefins on transition-metal-catalysed cross-coupling reactions. Angew. Chem. Int. Ed. 47, 840–871 (2008).
Findlater, M. et al. Role of coordination geometry in dictating the barrier to hydride migration in d6 square-pyramidal iridium and rhodium pincer complexes. J. Am. Chem. Soc. 133, 12274–12284 (2011).
Morales-Morales, D. Pincer complexes. Applications in catalysis. Rev. Soc. Quím. Méx. 48, 338 (2008).
Benito-Garagorri, D. & Kirchner, K. Modularly designed transition metal PNP and PCP pincer complexes based on aminophosphines: synthesis and catalytic applications. Acc. Chem. Res. 41, 201–213 (2008).
Qiu, X., Wang, M., Zhao, Y. & Shi, Z. Rhodium(I)-catalysed tertiary phosphine directed C-H arylation: rapid construction of ligand libraries. Angew. Chem. Int. Ed. 56, 7233–7237 (2017).
Crawford, K. M., Ramseyer, T. R., Daley, C. J. A. & Clark, T. B. Angew. Chem. Int. Ed. 53, 7589 (2014).
Wright, S. E. et al. Accessing ambiphilic phosphine boronates through C–H borylation by an unforeseen cationic iridium complex. Angew. Chem. Int. Ed. 58, 2834–2838 (2019).
Bedford, R. B., Coles S. J., Hursthouse, M. B. & Limmert, M. E. The catalytic intermolecular ortho-arylation of phenols. Angew. Chem. Int. Ed. 42, 112–114 (2003).
Yang, J.-F. et al. Ligand-accelerated direct C-H arylation of BINOL: a rapid one-step synthesis of racemic 3,3’-diaryl BINOLs. Angew. Chem. Int. Ed. 55, 14116 (2016).
Qiu, X., Deng, H., Zhao, Y. & Shi, Z. Rhodium-catalysed, P-directed selective C7 arylation of indoles. Sci. Adv. 4, 6468 (2018).
Borah, A. J. & Shi, Z. Rhodium-catalysed, remote terminal hydroarylation of activated olefins through a long-range deconjugative isomerization. J. Am. Chem. Soc. 140, 6062–6066 (2018).
Qiu, X. et al. PIII-chelation-assisted indole C7-arylation, olefination, methylation, and acylation with carboxylic acids/anhydrides by rhodium catalysis. Angew. Chem. Int. Ed. 58, 1504–1508 (2019).
Wen, J. et al. Rhodium-catalysed PIII-directed ortho-C−H borylation of arylphosphines. Angew. Chem. Int. Ed. 58, 2078–2082 (2019).
Fukuda, K., Iwasawa, N. & Takaya, J. Ruthenium-catalysed ortho C-H borylation of arylphosphines. Angew. Chem. Int. Ed. 58, 2850–2853 (2019).
Ma, Y.-N., Li, S.-X. & Yang, S.-D. New approaches for biaryl-based phosphine ligand synthesis via P=O directed C-H functionalizations. Acc. Chem. Res. 50, 1480–1492 (2017).
Wang, H.-L. et al. Pd(II)-catalysed Ph2(O)P-directed C–H olefination toward phosphine–alkene ligands. Org. Lett. 15, 5302–5305 (2013).
Li, S.-X., Ma, Y.-N. & Yang, S.-D. P(O)R2-directed enantioselective C–H olefination toward chiral atropoisomeric phosphine–olefin compounds. Org. Lett. 19, 1842–1845 (2017).
Shelby, Q., Kataoka, N., Mann, G. & Hartwig, J. Unusual in situ ligand modification to generate a catalyst for room temperature aromatic C-O bond formation. J. Am. Chem. Soc. 122, 10718–10719 (2000).
Lu, S., Poh, S. B., Siau, W.-Y. & Zhao, Y. Kinetic resolution of tertiary alcohols: highly enantioselective access to 3-hydroxy-3-substituted oxindoles. Angew. Chem. Int. Ed. 52, 1731–1734 (2013).
Shintani, R., Inoue, M. & Hayashi, T. Rhodium-catalysed asymmetric addition of aryl- and alkenylboronic acids to isatins. Angew. Chem. Int. Ed. 45, 3353–3356 (2006).
O’Connor, A. R., Kaminsky, W., Heinekey, D. M. & Goldberg, K. I. Synthesis, characterization, and reactivity of arene-stabilized rhodium complexes. Organometallics 30, 2105–2116 (2011).
Liu, Z., Yamamichi, H., Madrahimov, S. T. & Hartwig, J. F. Rhodium phosphine−π-arene intermediates in the hydroamination of alkenes. J. Am. Chem. Soc. 133, 2772–2782 (2011).
van der Boom, M. E., Higgitt, C. L. & Milstein, D. Directly observed β-H elimination of unsaturated PCP-based Rhodium(III)−alkyl complexes. Organometallics 18, 2413 (1999).
Padwa, A. & Xu, S. L. A new phenol synthesis from the rhodium (I)-catalysed reaction of cyclopropenes and alkynes. J. Am. Chem. Soc. 114, 5881–5882 (1992).
Acknowledgements
We thank the “1000-Youth Talents Plan”, the National Natural Science Foundation of China (Grants 21672097) and the “Innovation & Entrepreneurship Talents Plan” of Jiangsu Province for their financial support.
Author information
Authors and Affiliations
Contributions
D.W., B.D., Y.W., J.Q. and J.Z. performed the experiments. Y.Z. performed the crystallographic studies. Z.S. conceived the concept, directed the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Communications thanks anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Dong, B., Wang, Y. et al. Rhodium-catalysed direct hydroarylation of alkenes and alkynes with phosphines through phosphorous-assisted C−H activation. Nat Commun 10, 3539 (2019). https://doi.org/10.1038/s41467-019-11420-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-11420-5
This article is cited by
-
Atroposelective hydroarylation of biaryl phosphines directed by phosphorus centres
Nature Communications (2023)
-
Rhodium-catalyzed selective direct arylation of phosphines with aryl bromides
Nature Communications (2022)
-
Ni-catalyzed hydroarylation of alkynes with unactivated β-C(sp2)−H bonds
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.