Abstract
Chiral aziridines are structure units found in many biologically active compounds and are important building blocks in organic synthesis. Herein, by merging nucleophilic generation through copper(I)-catalyzed decarboxylation and activation of poorly electrophilic 2H-azirines through protonation with carboxylic acids, an asymmetric decarboxylative Mannich reaction between α,α-disubstituted cyanoacetic acids and 2H-azirines is uncovered, which leads to generation of chiral aziridines containing vicinal tetrasubstituted and acyclic quaternary stereogenic carbon centers in good to excellent diastereo- and enantioselectivities. At last, transformations of the produced chiral aziridine are successfully carried out to deliver synthetically useful compounds.
Similar content being viewed by others
Introduction
Nature uses a mild but powerful strategy to generate a nucleophile through decarboxylation of a malonic acid half thioester in the presence of an enzyme, which is typically employed in the biosynthesis of fatty acids and polyketides1. Inspired by this fantastic method in nature, Shair and colleagues2 developed a Cu(II)-catalyzed asymmetric thioester aldol reaction, which was compatible with a broad range of protic functional groups and enolizable aldehydes due to the mild acidic reaction conditions2. Unfortunately, their following research uncovered that their reaction occurred by a different mechanism from the nature’s reaction (the nucleophile was actually not generated through decarboxylation)3. In 2009, Shibasaki and Kanai group4 mimicked the method in nature by using α-methyl-α-phenyl cyanoacetic acid as a pronucleophile and accomplished a decarboxylative Mannich-type reaction of N-diphenyl-phosphinoyl aldimines and a decarboxylative aldol reaction of aldehydes under copper(I) catalysis, which generated contiguous trisubstituted and acyclic quaternary stereocenters in moderate to high diastereo- and enantioselectivities4,5.
However, in the above two catalytic asymmetric decarboxylative reactions, cyanoacetic acid was only employed as the precursor for decarboxylative nucleophile generation6,7 by anion exchange with CuOAc–bisphosphine complex (decarboxylative protonation was a side reaction8). However, the acidic nature of cyanoacetic acid was not fully utilized. Considering the basicity and the poor electrophilicity of 2H-azirines, we propose a strategy that basic 2H-azirines might be activated by a cyanoacetic acid through protonation to afford more electrophilic iminium species (Fig. 1)9,10. Meanwhile, the anion exchange leads to the formation of copper(I) cyanoacetate, which affords the nucleophilic copper(I) ketenimide through extrusion of CO2. Then, asymmetric addition of the nucleophilic copper(I) ketenimide to the electrophilic iminium species would allow the easy formation of a series of chiral aziridines in good yields, which contain chiral adjacent tetrasubstituted and acyclic quaternary stereocenters. These chiral aziridines would serve as intermediates to furnish synthetically useful compounds through the transformations of the aziridine functional group.
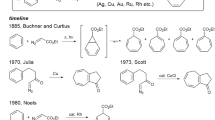
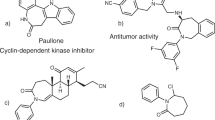
It is well known that chiral aziridines are important intermediates in organic synthesis, as they can react with nucleophiles to generate chiral amines through ring-opening reactions11,12,13,14,15,16. Moreover, chiral aziridines are structure units found in many natural products and man-made molecules, which exhibit a broad range of biological properties, such as antitumor and antibacterial activities17,18,19. Therefore, the asymmetric synthesis of chiral aziridines has received significant attention from the chemical community. One of the straightforward methods is the catalytic asymmetric addition of nucleophiles to 2H-azirines20,21. However, the lower reactivity of 2H-azirines has led to much less developments in this area22,23,24,25,26,27,28. Furthermore, it is well known that the asymmetric construction of vicinal chiral tetrasubstituted (including quaternary) and acyclic quaternary carbon stereocenters29,30,31 was very challenging and achieved much less success largely due to the significantly increased steric hindrance and the difficulty to control the asymmetric induction32,33,34,35.
Here, by activation of the poorly electrophilic 2H-azirines with carboxylic acids, we disclose a copper(I)-catalyzed decarboxylative Mannich reaction of 2H-azirines, which affords a series of chiral aziridines in good to high yields, diastereoselectivity, and enantioselectivity. More importantly, adjacent chiral tetrasubstituted and acyclic quaternary stereocenters are generated efficiently.
Results
Optimization of reaction conditions
The reaction between α-methyl-α-phenyl cyanoacetic acid (1a) and 2H-azirine 2a was studied for the optimization of reaction conditions as shown in Table 1. The decarboxylative Mannich reaction proceeded smoothly in the presence of 5 mol % of CuOAc and 5 mol % of (R)-BINAP at 0 oC in tetrahydrofuran (THF), which delivered product 3a in 81% yield with 2.0/1 dr and 8% ee for the major diastereoisomer (Table 1, entry 1). Screening of the commercially available bisphosphine ligands, including (R)-TOL-BINAP, (R)-SEGPHOS, (R)-DIFLUORPHOS, (R,R)-QUINOXP*, (R,R)-Ph-BPE, (R,RP)-TANIAPHOS, (R)-DTBM-SEGPHOS, and (R)-DIPA-MeO-BIPHEP, identified (R)-DTBM-SEGPHOS and (R)-DIPA-MeO-BIPHEP as the suitable ligands (Table 1, entries 2–9). With (R)-DTBM-SEGPHOS as the ligand, product 3a was generated in 88% yield with 4.0/1 dr and 93% ee for the major diastereoisomer (Table 1, entry 8). In the case of (R)-DIPA-MeO-BIPHEP, product 3a was obtained in 62% yield with 5.7/1 dr and 91% ee for the major diastereoisomer (Table 1, entry 9).
With (R)-DTBM-SEGPHOS as the ligand, the studies of copper(I) source, reaction solvent, and temperature effect were not fruitful (see Supplementary Table 1-3 for details). Fortunately, decreasing the reaction temperature to −20 oC resulted in improved yield, diastereo-, and enantioselectivities in the case of (R)-DIPA-MeO-BIPHEP (Table 1, entry 10). By further lowering the reaction temperature to −60 oC, product 3a was generated in 98% yield with >20/1 dr and 98% ee (Table 1, entry 11). Moreover, the loading of CuOAc-(R)-DIPA-MeO-BIPHEP complex was successfully reduced to 3 mol % by prolonging the reaction time from 24 h to 36 h (Table 1, entry 12). It was verified that both CuOAc and ligand ((R)-DIPA-MeO-BIPHEP) were indispensable, as no product 3a was observed in the absence of either (Table 1, entries 13, 14). The catalyst loading was further reduced to 2 mol % with the success of a gram-scale reaction as shown in Fig. 2. Remarkably, 1.358 g of aziridine 3a was isolated in 91% yield with >20/1 dr and 97% ee.
Substrate scope
The study of the substrate scope of aromatic 2H-azirines (2) with 3 mol % of CuOAc-(R)-DIPA-MeO-BIPHEP complex was described in Table 2. Both electron-donating groups and electron-withdrawing groups were accepted at the para-positon of the phenyl group (3a–3j). Although the diastereoselectivity was moderate in some cases, both the yield and the enantioselectivity were high to excellent. Several aromatic 2H-azirines with a substituent at the meta-position served as appropriate substrates to afford the aziridines in good to excellent stereoselectivities (3k–3n). Unfortunately, a substituent was not well tolerated at the ortho-position largely due to the increased steric hindrance. 2H-Azirine with a 2-naphthyl group was also a suitable substrate as excellent results were obtained (3o). Moreover, both 3-thienyl and benzo[b]thiophen-2-yl groups did not have a negative effect on the reaction results (3p and 3q).
Then, complex molecules containing an aromatic 2H-azirine moiety were investigated. 2,4-Dichlorophenoxyacetic acid, a systemic herbicide, was successfully attached to the 2H-azirine group through an ester linker (2r). 2r underwent the copper(I)-catalyzed decarboxylative Mannich reaction smoothly to afford product 3r in excellent both yield and stereoselectivity. By using the same linker, isoxepac acid (an advanced intermediate toward the synthesis of olopatadine, which is sold as a prescription eye drop), probenecid (a medication that is primarily used in treating gout and hyperuricemia), and gabapentin (a medication that is used primarily to treat seizures and neuropathic pain) were attached with the 2H-azirine moiety to generate 2s, 2t, and 2u. These three 2H-azirines reacted with 1a nicely to furnish the aziridines (3s, 3t, and 3u) in uniformly excellent yield, high diastereoselectivity, and excellent enantioselectivity. It was noted that a ketone unit, a tertiary sulfonate amide, and a secondary carbamate did not disturb both the reactivity and the stereoselectivity. Moreover, the 2H-azirine moiety was introduced to lithocholic acid (a natural steroid molecule) through the same linker to generate a complex 2H-azirine (2v), which also served as a competent substrate to furnish product 3v in excellent both yield and diastereoselectivities.
As (R)-DTBM-SEGPHOS led to better control of the diastereoselectivity than (R)-DIPA-MeO-BIPHEP (6/1 dr vs. 4/1 dr) in the reaction of aliphatic 2H-azirine 4a, (R)-DTBM-SEGPHOS was employed for the study of aliphatic 2H-azirines (Table 3). Although the diastereoselectivity was not satisfactory in the cases of 5a and 5b, both the yields and the enantioselectivity were excellent. The same situation was also observed in the reaction of α,β-unsaturated 2H-azirine 4c. The aryl group in α,α-disubstituted cyanoacetic acid (1) was successfully extended to para-methyl-phenyl (1b), para-chloro-phenyl (1c), and 2-thienyl groups (1d) with high to excellent stereoselectivities (5d, 5e, and 5f). It was noted that cyanoacetic acid 1c with a para-chloro-phenyl led to moderate yield. Moreover, the alkyl group in 1 was successfully changed from methyl (1a) to ethyl and allyl groups (1e and 1f). Unfortunately, the yields were moderate and the diastereoselectivity decreased significantly, possibly due to the extenuated difference of the steric hindrance between phenyl and ethyl or allyl groups (5g and 5h). Furthermore, the 2H-azirine moiety was tethered with indometacin (a nonsteroidal anti-inflammatory drug), dehydroepiandrosterone (an endogenous steroid hormone), and a protected glucose to give three complex aliphatic 2H-azirines (4i, 4j, and 4k). Their decarboxylative Mannich reactions with 1a proceeded smoothly to give the aziridines (5i, 5j, and 5k) in high yields with high to excellent stereoselectivities.
Demonstration of the strategy
The present decarboxylative Mannich reaction exhibited an impressive advantage over the classical proton-transfer version. As shown in Fig. 3, the proton-transfer Mannich reaction of 1a’ and 2a proceeded in <5% yield in the presence of 5 mol % of CuOAc-(R)-DIPA-MeO-BIPHEP complex (Fig. 3b), while the decarboxylative version occurred in 98% yield (Fig. 3a). Moreover, the retro-Mannich reaction of 3a in the presence of 5 mol % of CuOtBu and 5 mol % of (R)-DIPA-MeO-BIPHEP at −60 oC did not proceed at all, indicating that the very low yield of the proton-transfer Mannich reaction between 1a’ and 2a was not attributed to the retro-Mannich reaction of 3a under basic conditions. The same diastereo- and enantioselectivities were observed both in the decarboxylative version and in the proton-transfer version, indicating that the same copper(I) intermediate was generated. It is reasonable that the proton-transfer version led to very low yield as 2H-azirine 2a is a poor electrophile as described in literature25,28. As 2H-azirine 2a is an imine base, it is also reasonable that the protonation of 2a by 1a might occur smoothly to give an iminium cation, which is a highly electrophilic species. Moreover, it was observed that the copper(I)-catalyzed decarboxylation protonation of 1a proceeded very slowly in the absence of 2a at −60 oC, which indicated the interaction between 1a and 2a (the activation of 2a by 1a through a hydrogen-bonding effect can not be excluded completely at present). The difference of the electrophiles in the Mannich reaction resulted in different yields. The same tendency was also observed in the Mannich reaction of aliphatic 2H-azirine 4a with (R)-DTBM-SEGPHOS as the ligand (Fig. 3c, d). It is obvious that the present acidic reaction conditions are superior to the classical basic proton-transfer reaction conditions in the construction of contiguous tetrasubstituted and quaternary stereocenters.
Demonstration of the strategy―one substrate activation by the other. a Decarboxylative Mannich reaction of aromatic 2H-azirine. b Proton-transfer Mannich reaction of aromatic 2H-azirine. c Decarboxylative Mannich reaction of aliphatic 2H-azirine. d Proton-transfer Mannich reaction of aliphatic 2H-azirine
Transformations
Transformations of 3a were shown in Fig. 4. After protection of 3a with an acid chloride, the rearrangement of the aziridine moiety in 3a to an oxazoline group produced 6 in 63% yield with maintained enantioselectivity25.The reduction of cyano group by DIBAL-H afforded carbamate 7 in 60% yield after protection of the free amine with Boc2O. Moreover, the acidic opening of the aziridine group in 3a with HCl proceeded smoothly to furnish amine 8 in 80% yield. Obviously, the primary alkyl chloride moiety in 8 allows further structure elaboration. At last, the absolute configurations of the stereocenters in 8 were determined by the X-ray analysis of its single crystals as depicted in Fig. 4, which led to the determination of the exact stereochemistry of 3a. Then, the absolute configurations in 3 and 5 were assigned tentatively by analogy (see Supplementary Fig. 40 and Supplementary Table 4 for details).
Discussion
In conclusion, a copper(I)-catalyzed decarboxylative Mannich reaction between α,α-disubstituted cyanoacetic acids and various 2H-azirines was disclosed, which efficiently constructed chiral aziridines bearing vicinal tetrasubstituted and acyclic quaternary stereogenic carbon centers. The present reaction enjoyed the advantages of mild reaction conditions, easy reaction protocol, broad substrate scope, and high to excellent stereoselectivity. The activation of basic 2H-azirines by cyanoacetic acids, which might generate highly electrophilic iminium species, was proposed to be the key for the success of the present challenging reaction. At last, several transformations of the product were successfully carried out by means of the synthetically versatile cyano and aziridine groups. Expansion of both the present activation strategy and the mild nucleophile generation strategy through copper(I)-catalyzed decarboxylation in asymmetric catalysis is currently in progress in our laboratory.
Methods
General procedure for the synthesis of 3a
A dried 25 mL Schlenk tube equipped with a magnetic stirring bar was charged with CuOAc (2.94 mg, 0.024 mmol) and (R)-DIPA-MeO-BIPHEP (26.2 mg, 0.024 mmol) in a glove box under Ar atmosphere. Anhydrous THF (4 mL) was added via a syringe. The mixture was stirred for 15 min to give a clear catalyst solution. Another dried 25 mL Schlenk tube equipped with a magnetic stirring bar was charged with aromatic 2H-azirines 2a (0.2 mmol, 1.0 equiv, 23.4 mg). The catalyst solution (1.0 mL) containing copper(I) complex (0.006 mmol, 0.03 equiv) was added via a syringe. The reaction mixture was then cooled to −60 °C and cyanoacetic acid 1a (0.3 M in THF, 1.0 mL, 0.3 mmol, 1.5 equiv, 52.6 mg) was added dropwise over 2 min. The resulting reaction mixture was stirred at −60 °C for 36 h. The diastereomeric ratio of the crude reaction mixture was determined by 1H NMR spectroscopy. Then, the residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 5/1) to give 3a in 92% yield as pale yellow solid.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1891666 (8). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. The data supporting the findings of this study are available within the article and its Supplementary Information file. Any further relevant data are available from the authors on request.
References
Staunton, J. & Weissman, K. J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 18, 380–416 (2001).
Magdziak, D. et al. Catalytic enantioselective thioester aldol reactions that are compatible with protic functional groups. J. Am. Chem. Soc. 127, 7284–7285 (2005).
Fortner, K. C. & Shair, M. D. Stereoelectronic effects dictate mechanistic dichotomy between Cu(II)-catalyzed and enzyme-catalyzed reactions of malonic acid half thioesters. J. Am. Chem. Soc. 129, 1032–1033 (2007).
Yin, L., Kanai, M. & Shibasaki, M. Nucleophile generation via decarboxylation: asymmetric construction of contiguous trisubstituted and quaternary stereocenters through a Cu(I)-catalyzed decarboxylative Mannich-type reaction. J. Am. Chem. Soc. 131, 9610–9611 (2009).
Yin, L., Kanai, M. & Shibasaki, M. Cu(I)-catalyzed decarboxylative aldol-type and Mannich-type reactions for asymmetric construction of contiguous trisubstituted and quaternary stereocenters. Tetrahedron 68, 3497–3506 (2012).
Hyodo, K., Kondo, M., Funahashi, Y. & Nakamura, S. Catalytic enantioselective decarboxylative cyanoalkylation of imines by using palladium pincer complexes with C2-symmetric chiral bis(imidazoline)s. Chem. Eur. J. 19, 4128–4134 (2013).
Gajulapalli, V. P. R., Vinayagam, P. & Kesavan, V. Organocatalytic asymmetric decarboxylative cyanomethylation of isatins using L-proline derived bifunctional thiourea. Org. Biomol. Chem. 12, 4186–4191 (2014).
Brunner, H. & Schmidt, P. Naproxen derivatives by enantioselective decarboxylation. Eur. J. Org. Chem. 2000, 2119–2133 (2000).
Wang, Z.-L. Recent advances in catalytic asymmetric decarboxylative addition reactions. Adv. Synth. Catal. 355, 2745–2755 (2013).
Nakamura, S. Catalytic enantioselective decarboxylative reactions using organocatalysts. Org. Biomol. Chem. 12, 394–405 (2014).
Tanner, D. Chiral aziridines-their synthesis and use in stereoselective transformations. Angew. Chem. Int. Ed. Engl. 33, 599–619 (1994).
McCoull, W. & Davis, F. A. Recent synthetic applications of chiral aziridines. Synthesis 2000, 1347–1365 (2000).
Hu, X. E. Nucleophilic ring opening of aziridines. Tetrahedron 60, 2701–2743 (2004).
Pineschi, M. Asymmetric ring-opening of epoxides and aziridines with carbon nucleophiles. Eur. J. Org. Chem. 2006, 4979–4988 (2006).
Schneider, C. Catalytic, enantioselective ring opening of aziridines. Angew. Chem. Int. Ed. 48, 2082–2084 (2009).
Krake, S. H. & Bergmeier, S. C. Inter-and intramolecular reactions of epoxides and aziridines with π-nucleophiles. Tetrahedron 66, 7337–7360 (2010).
Yudin, A. K. Aziridines and Epoxides in Organic Synthesis (Wiley-VCH: Weinheim, 2006).
Ismail, F. M. D., Levitsky, D. O. & Dembitsky, V. M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 44, 3373–3387 (2009).
Singh, G. S. Synthetic aziridines in medicinal chemistry: a mini-review. Mini-Rev. Med. Chem. 16, 892–904 (2016).
Khlebnikov, A. F. & Novikov, M. S. Recent advances in 2H-azirine chemistry. Tetrahedron 69, 3363–3401 (2013).
Huang, C.-Y. & Doyle, A. G. The chemistry of transition metals with three-membered ring heterocycles. Chem. Rev. 114, 8153–8198 (2014).
Risberg, E. & Somfai, P. Enantioselective addition of organolithium reagents to a 2H-azirine. Tetrahedron Asymmetry 13, 1957–1959 (2002).
Hu, H. et al. Kinetic resolution of 2H-azirines by asymmetric imine amidation. Angew. Chem. Int. Ed. 55, 10098–10101 (2016).
An, D. et al. Organocatalyzed nucleophilic addition of pyrazoles to 2H-azirines: asymmetric synthesis of 3,3-disubstituted aziridines and kinetic resolution of racemic 2H-azirines. Chem. Commun. 52, 11211–11214 (2016).
Nakamura, S. & Hayama, D. Enantioselective reaction of 2H-azirines with phosphite using chiral bis(imidazoline)/zinc(II) catalysts. Angew. Chem. Int. Ed. 56, 8785–8789 (2017).
Peng, Q., Guo, D., Bie, J. & Wang, J. Catalytic enantioselective aza-benzoin reactions of aldehydes with 2H-azirines. Angew. Chem. Int. Ed. 57, 3767–3771 (2018).
Nakamura, S., Hayama, D., Miura, M., Hatanaka, T. & Funahashi, Y. Catalytic enantioselective reaction of 2H-azirines with thiols using cinchona alkaloid sulfonamide catalysts. Org. Lett. 20, 856–859 (2018).
Hu, H. et al. Copper-catalyzed asymmetric addition of tertiary carbon nucleophiles to 2H-azirines: access to chiral aziridines with vicinal tetrasubstituted stereocenters. Org. Lett. 20, 5601–5605 (2018).
Douglas, C. J. & Overman, L. E. Catalytic asymmetric synthesis of all-carbon quaternary stereocenters. Proc. Natl. Acad. Sci. USA 101, 5363–5367 (2004).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Feng, J., Holmes, M. & Krische, M. J. Acyclic quaternary carbon stereocenters via enantioselective transition metal catalysis. Chem. Rev. 117, 12564–12580 (2017).
Gonthier, J. F., Wodrich, M. D., Steinmann, S. N. & Corminboeuf, C. Branched alkanes have contrasting stabilities. Org. Lett. 12, 3070–3073 (2010).
Mitsunuma, H., Shibasaki, M., Kanai, M. & Matsunaga, S. Catalytic asymmetric total synthesis of chimonanthine, folicanthine, and calycanthine through double Michael reaction of bisoxindole. Angew. Chem. Int. Ed. 51, 5217–5221 (2012).
Marek, I. et al. All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C-C bonds per chemical step. J. Am. Chem. Soc. 136, 2682–2694 (2014).
Watson, C. G. et al. Construction of multiple, contiguous quaternary stereocenters in acyclic molecules by lithiation-borylation. J. Am. Chem. Soc. 136, 17370–17373 (2014).
Acknowledgements
We gratefully acknowledge the financial support from the “Thousand Youth Talents Plan”, the National Natural Science Foundation of China (No. 21672235 and No. 21871287), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000), CAS Key Laboratory of Synthetic Chemistry of Natural Substances, and Shanghai Institute of Organic Chemistry.
Author information
Authors and Affiliations
Contributions
L.Y. guided the research and wrote the manuscript. H.-J.Z. and Y.-C.X. performed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, HJ., Xie, YC. & Yin, L. Copper(I)-catalyzed asymmetric decarboxylative Mannich reaction enabled by acidic activation of 2H-azirines. Nat Commun 10, 1699 (2019). https://doi.org/10.1038/s41467-019-09750-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-09750-5
This article is cited by
-
Tyrosinase-mediated synthesis of larvicidal active 1,5-diphenyl pent-4-en-1-one derivatives against Culex quinquefasciatus and investigation of their ichthyotoxicity
Scientific Reports (2021)
-
2H-Azirines in medicinal chemistry
Chemistry of Heterocyclic Compounds (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.