Abstract
Ischemia causes an inflammatory response that is intended to restore perfusion and homeostasis yet often aggravates damage. Here we show, using conditional genetic deletion strategies together with adoptive cell transfer experiments in a mouse model of hind limb ischemia, that blood vessels control macrophage differentiation and maturation from recruited monocytes via Notch signaling, which in turn promotes arteriogenesis and tissue repair. Macrophage maturation is controlled by Notch ligand Dll1 expressed in vascular endothelial cells of arteries and requires macrophage canonical Notch signaling via Rbpj, which simultaneously suppresses an inflammatory macrophage fate. Conversely, conditional mutant mice lacking Dll1 or Rbpj show proliferation and transient accumulation of inflammatory macrophages, which antagonizes arteriogenesis and tissue repair. Furthermore, the effects of Notch are sufficient to generate mature macrophages from monocytes ex vivo that display a stable anti-inflammatory phenotype when challenged with pro-inflammatory stimuli. Thus, angiocrine Notch signaling fosters macrophage maturation during ischemia.
Similar content being viewed by others
Introduction
Growth of functional arteries is essential for the restoration of blood flow to ischemic limbs and organs. Ischemia causes an inflammatory response that is intended to initiate restoration of perfusion and tissue repair yet often aggravates damage or disturbs healing1, 2. Monocytes and macrophages in particular are recruited to ischemic vessels and tissues and are essential for remodeling of small collateral vessels into conduit arteries, or arteriogenesis, a process capable of fully restoring blood flow3.
Macrophages have diverse phenotypes and functions in different contexts, ranging from pro-inflammatory states with detrimental effects on tissues in host defense to trophic or reparative roles, as described in arteriogenesis4, 5. Macrophage diversity is considered to arise from combinations of differentiation and activation or polarization programs6. Differentiation programs induce a stable and irreversible transition and maturation from macrophage precursors to distinct macrophage lineages under the control of external lineage factors, such as macrophage colony stimulating factor (CSF1) or granulocyte-macrophage CSF (CSF2)7. These cooperate with mostly unknown tissue-specific identity signals6, which can ultimately lead to terminal macrophage differentiation mediated by Ets repressor Etv3 (METS), which induces cell cycle arrest by suppressing Myc8. In contrast, activation programs induce mostly reversible changes in response to cytokines or danger signals6. This plasticity was initially termed polarization9, describing essentially pro- and anti-inflammatory fates, but was recently revised into a spectrum model of activation, recognizing that the number of stimuli and resultant activation programs is not dichotomous, but rather more diverse10. In a model of myocardial infarction, it was recently shown that the subset of Ly6Chi monocytes is recruited to ischemic tissues and differentiates into macrophages, which is required for cardiac healing, since impaired differentiation lead to adverse cardiac remodeling11. Exactly how macrophage differentiation and function is regulated during ischemia and arteriogenesis still remains largely unknown.
Organ-specific endothelial cells (EC) exert angiocrine functions that are involved in homeostasis and tissue regeneration independent of blood flow12. After injury, instructive cues expressed by local EC orchestrate the injury response of tissue-resident or hematopoietic progenitor cells and fibroblasts during organ regeneration, which in part is mediated by endothelial-specific expression of Notch ligands such as Jagged-113,14,15.
Notch signaling is a cell to cell contact-dependent signaling pathway regulating vascular development, branching morphogenesis, and homeostasis16,17,18. Activation of Notch receptors is controlled by membrane-bound Notch ligands of the Jagged (Jag) and Delta-like (Dll) gene families, which show different Notch receptor binding affinities and tissue expression patterns, thereby controlling specific Notch signaling outcomes19. The Notch ligand Dll1 is selectively expressed in vascular EC, and Dll1 haploinsufficiency leads to severely impaired arteriogenesis and ischemic tissue damage in a mouse model of hind limb ischemia20. We recently described that Notch signaling activated by endothelial Dll1 regulates cell fate of monocyte subsets under steady state conditions21.
We now tested the hypothesis that endothelial Dll1 mediates angiocrine effects in arteriogenesis by influencing macrophages in a mouse model of hind limb ischemia22. We show that endothelial Dll1 regulates macrophage differentiation and maturation from invading Ly6Chi monocytes, which promotes arteriogenesis and tissue repair after ischemia.
Results
Macrophages develop from Ly6Chi monocytes in ischemia
To study the origin and regulation of macrophages in response to peripheral tissue ischemia, we first characterized the population dynamics of monocytes and macrophages by flow cytometry (for gating and antibody panel see Supplementary Fig. 1 and Supplementary Table 1) in the mouse model of hind limb ischemia (HLI)22. We analyzed Cx3cr1 GFP/+ reporter mice23, in which monocyte and macrophages, but not granulocytes, express distinct intensities of GFP (mouse strain information see Supplementary Table 2). Ischemia triggered recruitment of Ly6Chi monocytes into muscle, which was closely followed by the appearance of macrophages (Fig. 1a, Supplementary Fig. 1). While the monocyte response was transient, macrophage development was more sustained and characterized by gradual down regulation of Ly6C and up regulation of Cx3cr1, CD11c, and MHC class II over time, suggesting maturation of macrophages from monocytes.
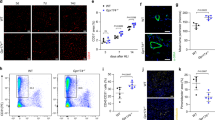
Origin of macrophages in ischemia. a Representative flow cytometric analysis of ischemic tibialis anterior muscle of Cx3cr1 GFP/+ mice. Granulocytes (green), Ly6Chi monocytes (blue) and macrophages (black), n = 3 mice/group. b Adoptive cell transfer at d1 after HLI and cell fate tracking of CD45.2+CD11b+GFP+Ly6Chi monocytes transferred into CD45.1+ recipients. Representative flow cytometry of recipient muscle, replicated at least three times. c Treatment with CCR2 blocking antibody MC-21 (α) or isotype control c for indicated time intervals (r × d) after HLI and analysis of cell populations in peripheral blood (PB) and muscle (M) by flow cytometry. n = 5/7/7 mice/group, error bars represent s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05 *indicates comparison between treatment with MC-21 (α) vs isotype control c by one way ANOVA with Bonferroni multiple comparison test. d Quantitative RT-PCR analysis of cell populations of Cx3cr1 GFP/+ mice sorted from d3 ischemic muscle relative to bone marrow (BM) Ly6Chi monocytes. n = 3 independent experiments, error bars represent s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05, #Comparison between muscle and BM Ly6Chi cells, *Comparison between muscle Ly6Chi and muscle macrophages (MF), one way ANOVA with Bonferroni multiple comparison post-test
After myocardial infarction, cardiac macrophages are reported to develop from Ly6Chi monocytes and mediate healing11. To confirm and extend these findings we performed adoptive transfer studies with Ly6Chi monocytes that were isolated from CD45.2+ Cx3cr1 GFP/+ mice (Supplementary Fig. 2a) and intravenously transferred into CD45.1+ recipient mice 1 day after induction of hindlimb ischemia. Cell fate of donor cells, distinguished from recipient by expression of GFP and congenic CD45, was analyzed by flow cytometry 1, 2, and 6 days after transfer in ischemic limb muscle. Transferred Ly6Chi monocytes gradually and uniformly adopted a macrophage phenotype similar to endogenous macrophages, displaying gradual downregulation of Ly6C and upregulation of F4/80, Fcγ receptor CD64 and CD11c integrin over time (Fig. 1b, Supplementary Fig. 2b). Thus, in ischemic muscle Ly6Chi monocytes give rise to macrophages, which continue to undergo phenotypic changes suggesting macrophage maturation over time. In contrast, donor monocytes retrieved from the spleen of recipient mice predominantly adopted a Ly6Clo monocyte phenotype, as previously reported21, which was not even transiently observed in muscle (Supplementary Fig. 2c, d).
Our data so far suggested that, in ischemic muscle, a wave of early invading Ly6Chi monocytes gives rise to macrophages that gradually change their phenotype as they differentiate. We therefore tested the effects of Ly6Chi monocyte-depletion on the development of ischemic macrophages using anti-CCR2 antibody treatment (clone MC-21), which selectively and transiently depletes Ly6Chi monocytes24. We specifically addressed the effects of early vs. delayed monocyte depletion on the kinetics of macrophage development after ischemia. With an early depletion strategy (d0–d4 after HLI), MC-21 treatment strongly depleted Ly6Chi monocytes from peripheral blood (PB) and muscle at day 3 and also dramatically reduced the number of macrophages at this time point (Fig. 1c). At d7, Ly6Chi monocyte levels in PB recovered, reflecting the transient nature of monocyte depletion, while Ly6Chi monocyte numbers in muscle were increased but numbers of macrophages were still strongly reduced compared with isotype control (Fig. 1c). In contrast, delayed antibody treatment (from d4 onwards) reduced the number of Ly6Chi monocytes in PB, but did not significantly affect the number of ischemic macrophages at day 7. Thus, while initial depletion of Ly6Chi monocytes impairs early and late macrophage populations, delayed monocyte depletion does not influence macrophage development. These data clearly demonstrate that early invading Ly6Chi monocytes give rise to ischemic macrophages, which continue to change their phenotype as they develop over time.
We next characterized the gene expression changes occurring during monocyte-to-macrophage differentiation in ischemic muscle. We isolated Ly6Chi monocytes and macrophages from ischemic muscle and compared the gene expression signatures with naive Ly6Chi monocytes from the bone marrow (BM) of the same mouse. Compared with BM Ly6Chi monocytes, ischemic muscle Ly6Chi monocytes showed significant upregulation of transcription factor Mafb, involved in macrophage differentiation from precursors, and the macrophage marker Mer tyrosine kinase receptor (Mertk) (Fig. 1d)25. On the other hand, compared with muscle monocytes, ischemic macrophages showed significantly elevated levels of genes involved in macrophage differentiation (Mafb, Scavenger receptor Sra) and maturation, indicated by suppression of Myc but high expression of the transcription factor Etv3, which regulates terminal macrophage differentiation by suppressing Myc (Fig. 1d)8. At the same time, macrophages showed upregulation of Mannose receptor Mrc1 and Mertk, and high expression of anti-inflammatory Il10 and low expression of inflammatory cytokines Il6 and Il12, a profile consistent with reparative macrophages. Altogether, these data demonstrate that ischemic macrophages develop from Ly6Chi monocytes by gradual differentiation and maturation.
Dll1 promotes macrophage differentiation
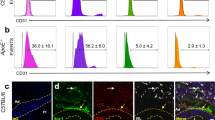
We also compared expression patterns of Notch target genes in BM monocytes and ischemic monocytes and macrophages. The Notch target genes Hey1 and Hes1 were markedly upregulated in cells isolated from ischemic muscle, showing the highest expression levels in macrophages (Fig. 2a). This indicates active Notch signaling during macrophage differentiation in ischemic tissues. At the same time, expression of the Notch ligand Delta-like 1 (Dll1) was increased in ischemic muscle with a timecourse matching the appearance of macrophages (Fig. 2b). Analysis of ischemic muscle sections of Dll1 +/lacZ reporter mice confirmed our previous observation that Dll1 expression is restricted to arteries (Fig. 2c)20. To capture a possible interaction between macrophages and blood vessels we performed HLI in Cx3cr1 GFP/+ reporter mice, followed by in vivo perfusion fixation and confocal microscopy. This revealed adhesion of GFP+ cells to the inside of growing collateral arteries at d3 after HLI, the period of highest Dll1 expression and macrophage induction (Fig. 2d, Supplementary Fig. 3).
Ischemia triggers induction of Notch ligand Dll1 in arteries. a Quantitative RT-PCR analysis of cell populations of Cx3cr1 GFP/+ mice sorted from d3 ischemic muscle relative to bone marrow (BM) Ly6Chi monocytes. n = 3 independent experiments, error bars represent s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05 #Comparison between muscle and BM Ly6Chi cells, *Comparison between muscle Ly6Chi and macrophages (MF), One way ANOVA with Bonferroni multiple comparison post-test b Quantitative RT-PCR analysis of ischemic muscle at indicated time points d. n = 3/4/4/4 mice/group, error bars represent s.e.m., one way ANOVA with Dunnett's Multiple Comparison test. c β-galactosidase activity (blue) and CD45 immunohistochemistry (red) in ischemic muscle from Dll1 +/lacZ reporter mice. Scale bars=50 µm. d Representative immunostaining and confocal microscopy of collateral arteries in Cx3cr1 GFP/+ mice after HLI. Phase contrast image (GFP) × 5, scale bar=200 µm. Confocal images with anti-SMA × 40, CD31 × 40 and × 63. Nuclear counterstain (DAPI, blue). Scale bar=20 µm
Our in vivo data suggested that Dll1-activated Notch signaling is involved in ischemic macrophage differentiation from monocytes. To test this under defined conditions in vitro we cultured Ly6Chi monocytes from Cx3cr1 GFP/+ reporter mice on recombinant DLL1. Compared with control conditions, DLL1-activated Notch signaling in culture (Supplementary Fig. 4a) and generated CD11b+F4/80+CD115+ macrophages that showed upregulation of GFP, CD11c, and MHC class II, a phenotype resembling ischemic tissue macrophages (Fig. 3a, Supplementary Fig. 4b, c). In contrast, the ligand JAG1 was significantly less efficient in generating this phenotype (Supplementary Fig. 4d). CSF1 was required for these effects, as culture without CSF1 resulted in low-cell yield, while culture of monocytes with CSF2 resulted in complete downregulation of F4/80 and GFP regardless of ligand stimulation (Supplementary Fig. 5a, b). Furthermore, cells cultured on DLL1 in the presence of CSF1 upregulated Mertk and expressed CD64 and TLR4 (Fig. 3b, Supplementary Fig. 4e), core macrophage markers, but not the dendritic cell lineage factor Zbtb46 26, indicating macrophage cell fate. In contrast, culture with CSF2 induced Zbtb46, but not Mertk, regardless of ligand stimulation (Fig. 3b).
DLL1 instruct macrophage maturation in vitro. a Flow cytometry of Ly6Chi monocytes from Cx3cr1 GFP/+ mice cultured for 3d on IgG-Fc (con) or DLL1-Fc chimeric proteins, in the presence of 10 ng/ml CSF1. Representative of n = 5 independent experiments. b Quantitative RT-PCR analysis at d3 after culture with CSF1, CSF2. n = 2 independent experiments performed in duplicates, error bars represent s.e.m. **p < 0.01, *p < 0.05 by one way ANOVA with Bonferroni multiple comparison post-test. c Representative analysis of proliferation by 6 h BrdU incorporation at d3. n = 3 independent experiments. d GFP+ cell numbers in culture relative to input number. n = 3 independent experiments performed in duplicates, error bars represent s.e.m. **p < 0.01 by two-way ANOVA and Bonferroni multiple comparison post-test. e Quantitative RT-PCR analysis normalized to gene expression levels of input Ly6Chi monocytes (d0). n = 4 independent experiments performed in duplicates, error bars represent s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05 by two way ANOVA with Bonferroni multiple comparison post-test. f BrdU incorporation in CD11b+ cells at day 7 after 100 ng/ml CSF1 pulse. n = 3 independent experiments, error bars represent s.e.m. ***p < 0.001 by Student’s unpaired t-test. g Representative Annexin V/7-AAD staining gated on CD11b+ macrophages, n = 3 independent experiments, error bars represent s.e.m, **p < 0.01, Student’s unpaired t test. h Representative flow cytometry of phagocytosis (1 h) of PE-labeled dextran beads at indicated temperature, d3 of culture. n = 3 independent experiments, error bars represent s.e.m, *p < 0.05, Student’s unpaired t test. i Multiplex measurement of cytokine concentration in culture supernatant after stimulation with 100 ng/ml LPS (6 h) or combination of 20 ng/ml IFNγ (18 h) and LPS (6 h). n = 4 independent experiments, error bars represent s.e.m. **p < 0.01, *p < 0.05, Student’s paired t test. j, k Quantitative RT-PCR analysis after stimulation with IFNγ/LPS normalized to input monocyte levels. Comparison of cells stimulated j at d3 or d6, k with or without re-stimulation with CSF1 (100 ng/ml) at d6. n = 4 independent experiments in duplicates, error bars represent s.e.m. *p < 0.05 by Student’s paired t-test
Interestingly, DLL1-generated macrophages mostly remained in the G0/G1 phase of the cell cycle, had suppressed levels of cyclin-dependent kinases Cdk1 and Cdk2 and maintained steady cell numbers, while control macrophages showed a high-proliferation rate leading to transient expansion of macrophages in culture (Fig. 3c, d, Supplementary Fig. 4f). Of note, the effects of DLL1 were not explained by changes in surface expression of the CSF1 receptor (CD115, Supplementary Fig. 4b), but were blocked by the γ-secretase inhibitor DAPT, which inhibits Notch receptor activation (Supplementary Fig. 6). Furthermore, DLL1-induced upregulation of macrophage differentiation markers Mafb and Sra1, but also marked upregulation of the terminal differentiation mediator Etv3, and suppression of its downstream repression targets, Ets2 and Myc, while the macrophage master regulator Spi1 was not influenced (Fig. 3e). Our data thus suggested that DLL1 induces a switch from proliferation to differentiation of macrophages, indicating a role in macrophage maturation. A hallmark of (terminally) differentiated cells is cell cycle arrest in response to growth factor stimulation8. To test whether DLL1 promoted terminal macrophage differentiation we re-stimulated cells after 7 days of culture with a CSF1 pulse and quantified the proliferation rate. CSF1 induced proliferation in the majority of control macrophages, while only a small fraction of DLL1-cultured macrophages started to proliferate (Fig. 3f). In addition, DLL1-induced maturation was associated with decreased apoptosis during prolonged culture, contributing to stable cell numbers compared with cell loss observed in immature macrophages (Fig. 3d, g). In functional assays, DLL1 stimulated cells were also found to be more efficient phagocytes (Fig. 3h). Altogether, these data demonstrate a DLL1-induced switch between proliferation and macrophage differentiation.
We next characterized the macrophage inflammatory response. Stimulation of control macrophages with the LPS or LPS/IFN-γ combination, prototypical inflammatory stimuli, induced high secretion of pro-inflammatory cytokines, IL-6, IL-12, and CXCL1, but low levels of the anti-inflammatory cytokine IL-10, which is in line with the current paradigm of macrophage polarization. In contrast, DLL1-generated macrophages secreted much lower levels of pro-inflammatory cytokines, but very high levels of IL-10 (Fig. 3i). These results were confirmed on the level of messenger RNA (mRNA) profiles, which also revealed upregulation of Arg1 and strong suppression of inducible nitric oxide synthase (Nos2), Cxcl1, and Cxcl2 by DLL1 (Supplementary Fig. 7). Thus, DLL1 promotes macrophage maturation, which results in enhanced phagocytic capacity and anti-inflammatory activation even in response to pro-inflammatory stimuli. To test the principle association between maturation and anti-inflammation without the influence of DLL1 we first compared the inflammatory response of early (d3) vs. mature macrophages (d6), as macrophage differentiation progresses over time (Fig. 3d, Supplementary Fig. 8). When stimulated with LPS/IFN-γ, mature macrophages produced less inflammatory cytokines (Fig. 3j). In contrast, pulsing mature macrophages with CSF1 induces downregulation of the differentiation regulator Etv3, proliferation, and increased inflammatory responses in response to LPS/IFN-γ (Fig. 3f, k). Altogether, these data demonstrate that macrophage maturation influences the inflammatory response.
Endothelial cells regulate macrophage differentation in vitro
Our in vivo observations suggested that ischemia generates a transient niche of Dll1 expressed by arterial EC. Studies with cultured human primary EC from different vascular domains confirmed that arterial EC expressed higher levels of DLL1 compared with microvascular ECs (Fig. 4a), while levels of Notch ligands DLL4 and JAG1 were comparable (Supplementary Fig. 9a). As EC have been shown to promote macrophage polarization27, 28, we tested the hypothesis that EC induce macrophage maturation via DLL1. We first conducted co-culture experiments of human arterial EC together with human PB CD14+ CD16− monocytes, the human equivalent of murine Ly6Chi monocytes29, 30, and compared the phenotype to original donor monocytes (d0 CD14+) or control culture. At d3, co-cultured CD11b+ cells expressed high levels of CD16, CD163, CX3CR1, and CD209, a signature consistent with anti-inflammatory macrophages (Fig. 4b, Supplementary Fig. 10)31.
Endothelial Dll1 instructs macrophage maturation in vitro. a Quantitative RT-PCR analysis of p3 primary human aortic EC (HAEC, A) or coronary microvascular (MV) EC. n = 3 independent samples in duplicates/group, error bars represent s.e.m., **p < 0.01, Student’s unpaired t-test. b Representative flow cytometry from d3 culture of human CD14+ monocytes in the presence of 10 ng/ml M-CSF or HAEC (AEC). n = 4 independent experiments. c, d HAEC were transfected with anti-DLL1 siRNA (Si-DLL1) or scambled control (Scr) before co-culture with CD14+ monocytes. c. Quantitative RT-PCR analysis of CD11b+ isolated from co-culture normalized to expression levels of input monocytes, n = 3 independent experiments, error bars represent s.e.m. **p < 0.01, *p < 0.05, Student’s paired t-test. d BrdU incorporation (6 h) in CD11b+ cells from co-culture, n = 3 independent experiments performed with duplicate measurements, error bars represent s.e.m. *p < 0.05, Student’s unpaired t-test. e Protein concentration of CSF1 in co-culture supernatants (A: arterial EC, MV: microvascular EC) or macrophage (MF) supernatants by ELISA, nd: not detectable. n = 3 independent experiments performed in triplicates, error bars represent s.e.m. ***p < 0.001 one-way ANOVA with Bonferroni post-test. f Number of CD11b+ macrophages recovered after co-culture of CD14+ monocytes with HAEC treated with isotype (α-Con) and CSF1 neutralizing (α-CSF1) antibody. n = 2 independent experiments performed in triplicates, error bars represent s.e.m. p < 0.0001, Student’s unpaired t-test g, h Effect of HAEC (A) or MVEC (MV) on CD14+ monocytes. g Representative flow cytometry and profile of gated population after d3 of co-culture. n = 3 independent experiments. h Transcriptional profile of CD11b+ cells isolated after 3d of culture with EC (A vs MV) or CSF1, normalized to expression levels of input monocytes (d0). n = 3 independent experiments, error bars represent s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05, one way ANOVA with Bonferroni multiple comparison test
To address the role of endothelial Dll1 in macrophage maturation we pretreated EC with a DLL1-specific siRNA, which efficiently reduced DLL1 expression without altering expression of other Notch ligands or Notch target genes (Supplementary Fig. 11a, b). Gene expression analysis of CD11b+ macrophages retrieved from co-culture revealed suppressed levels of the Notch-signaling targets HES1, HEY1, but also suppression of genes associated with macrophage (terminal) differentiation, ETV3 and MAFB, concomitant with reduced surface expression CD163 (Supplementary Fig. 11c). In addition, expression of IL10 was reduced, while expression of IL8 was increased (Fig. 4c). Furthermore, DLL1 knockdown in EC also lead to a substantial increase in macrophage proliferation, indicating that Dll1 suppresses proliferation and promotes maturation of human macrophages (Fig. 4d).
Arterial EC expressed and secreted significantly more CSF1 than microvascular EC, which in contrast expressed higher levels of CSF2 (Fig. 4e, Supplementary Fig. 9b). Furthermore, secretion of CSF1 was required to generate mature macrophages on arterial EC, as macrophage generation was greatly inhibited by a CSF1-neutralizing antibody (Fig. 4f). We next reasoned that the observed differences in Dll1 expression and myeloid growth factor profiles in the two EC populations should have functional consequences for macrophage instruction. In co-culture experiments comparing arterial vs. microvascular EC, only arterial EC induced differentiation of CD14+ monocytes to CD16+ CD163+ macrophages (Fig. 4g). Furthermore, macrophages generated on arterial EC showed significantly higher levels of HES1 and MAFB, and significantly reduced levels of MYC, when compared with either macrophages generated in the presence of microvascular EC or CSF1 (Fig. 4h). Furthermore, the terminal differentiation regulator ETV3 was only induced in macrophages generated on arterial EC. Altogether, these data demonstrate that human arterial EC have the distinct ability to instruct macrophage differentiation and maturation from monocytes, which requires Dll1.
EC Dll1 regulates macrophage maturation and arteriogenesis in vivo
To test the hypothesis that endothelial Dll1 regulates macrophage maturation after ischemia in vivo we used endothelial-specific and inducible Dll1 mutant mice (Dll1 i∆EC), which carry conditional Dll1 alleles and the Cdh5(PAC)-CreERT2 transgene21 and which enable targeting under ischemic conditions (Supplementary Fig. 12a, b). Myeloid cell populations were analyzed by flow cytometry with a non-GFP-based strategy32, which gave similar results compared with the GFP-based strategy (Supplementary Fig. 13)21. Inducible Dll1 deletion did not change baseline peripheral leukocyte counts (Supplementary Fig. 14a). Following HLI, ischemic muscle recruitment of Ly6Chi monocytes or granulocytes was not significantly altered in conditional Dll1 mutant mice, as were PB leukocyte counts (Fig. 5a, Supplementary Fig. 14b) and expression levels of other Notch ligands (Supplementary Fig. 14c). However, macrophage differentiation kinetics was substantially altered in Dll1 i∆EC mutant mice, showing increased macrophage numbers initially, followed by a pronounced cell loss later (Fig. 5a). Gene expression analysis from macrophages isolated from ischemic muscle of Dll1 i∆EC mutant mice revealed significant reductions in macrophage differentiation genes, downregulation of Il10 and Hey1, but upreguation of inflammatory genes Il6 and Il12 (Fig. 5b). Thus, endothelial Dll1 regulates macrophage differentiation and inflammatory signature in vivo.
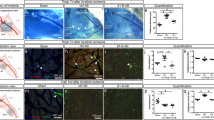
Endothelial Dll1 regulates macrophage maturation and arteriogenesis. a Analysis of cell populations by flow cytometry in ischemic muscle of induced endothelial Dll1 mutant mice (Dll1 iΔEC) and control mice. n = 8 mice/group, error bars represent s.e.m. *p < 0.05 by two way ANOVA with Bonferroni multiple comparison test. b Gene expression analysis of macrophages sorted from d3 ischemic muscle. n = 3 independent experiments, error bars represent s.e.m. **p < 0.01, *p < 0.05, Student’s unpaired t-test. c–f Rescue of endothelial Dll1 deficiency after HLI by transfer of ischemic macrophages. On d1 after HLI, indicated groups received intramuscular limb injections of 5 × 10e5 macrophages isolated from d3 ischemic muscle (MF) or Ly6Chi monocytes isolated from spleen (Mo) of wt mice, or received control treatment. c Foot perfusion by laser Doppler perfusion measurement immediately after induction of HLI (0) and during follow up. n = 5/7/7/7 mice/group, error bars represent s.e.m. ***p < 0.001 by two way ANOVA and Bonferroni post test. d Quantification of inner circumference and wall area, and representative H&E stained images of collateral arteries d14. Scale bar=50 μm. n = 7 mice/group, error bars represent s.e.m. ***p < 0.001 vs control by one way ANOVA and Dunnett’s multiple comparison test. e Representative SMA-stained immunofluorescence of muscle sections, Scale bar 100 μm (left) and quantification of arterial branches (SMA+) per section d14, n = 5 mice/group, error bars represent s.e.m. ***p < 0.001 vs control by one way ANOVA and Dunnett’s multiple comparison test. f Representative H&E stained muscle sections. Scale bar=50 μm (left) and quantification of ghost cells and regenerating muscle fibers (right) at d14, n = 5 mice/group, error bars represent s.e.m. ***p < 0.001 vs control by one way ANOVA and Dunnett’s multiple comparison test
We next addressed the relevance of endothelial Dll1 for arteriogenesis and ischemic muscle recovery22. To also address the role of Dll1-dependent macrophage differentiation we designed cell transfer experiments in which either peripheral Ly6Chi monocytes or d3 ischemic muscle macrophages from control donor mice were injected into ischemic limb muscles 1 day after HLI (Supplementary Fig. 15a, b). Hind limb perfusion measurements by laser Doppler imaging showed an impairment in foot perfusion in Dll1 i∆EC mutant mice, which was improved by transfer of macrophages, but not by transfer of monocytes (Fig. 5c). Histomorphometric analysis of arteriogenesis after HLI showed a significant impairment in luminal and wall growth in collaterals of Dll1 i∆EC mutant mice, which again was rescued by transfer of macrophages, but not monocytes (Fig. 5d), while Dll1 i∆EC mutant mice had no baseline collateral phenotype (Supplementary Fig. 16a). As ink perfusion studies also indicated post-ischemic branching defects in Dll1 i∆EC mutant mice (Supplementary Fig. 16b)22 we quantified the number of smooth muscle actin (SMA)+ collaterals in ischemic muscle. There was a significant reduction in collateral branch formation in Dll1 i∆EC mutant mice, which was again rescued by transfer of macrophages, but not monocytes (Fig. 5e). Analysis of capillary density, in contrast, showed an inverse response compared with collateral number, suggesting pronounced ischemia in conditions with reduced collaterals (Supplementary Fig. 16c).
Furthermore, ischemic muscle regeneration was severely impaired in Dll1 i∆EC mutant mice, indicated by a substantial increase in ghost cells and a significant reduction in regenerating muscle fibers, which was reversed by transfer of macrophages, but not monocytes (Fig. 5f). Taken together, these results suggest that blood vessel restricted Dll1 regulates macrophage maturation and activation state, a function required for arteriogenesis and ischemic tissue regeneration.
Canonical Notch signaling regulates macrophage maturation
We next asked whether Notch signaling in macrophages regulates ischemic macrophage differentiation and tissue recovery in vivo. We generated mice with conditional ablation of Notch signaling in macrophages (Rbpj ΔM, Supplementary Fig. 17a) to HLI, which were generated by crossing conditional alleles of the canonical Notch effector Rbpj 33, a myeloid specific Cre-recombinase, LysM Cre 34, and the Cx3cr1 GFP/+ reporter. Mice with conditional deletion of Rbpj had no alteration in circulating granulocytes or Ly6Chi monocyte numbers at baseline or following HLI, and no alteration in ischemic muscle recruitment (Fig. 6a, Supplementary Fig. 17b). However, differentiation of macrophages was severely altered in conditional Rbpj mutant mice, which showed increased numbers of proliferating macrophages (Fig. 6a, b) with impaired upregulation of CD11c and GFP (Fig. 6c). Moreover, gene expression analysis revealed impaired expression of genes involved in macrophage maturation (Mafb, Sra), terminal differentiation (Etv3), and antiinflammation (Mrc1, Arg1 and Il10), but upregulation of pro-inflammatory Il12 (Fig. 6d). Notch-deficiency also resulted in pronounced macrophage loss and increased macrophage cell death at later ischemic timepoints (Fig. 6a, e). Altogether, this phenocopies the kinetics observed in vitro and in conditional Dll1 mutant mice in vivo.
Canonical Notch signaling regulates macrophage maturation in vivo. a Analysis of cell populations by flow cytometry in ischemic muscle of Rbpj conditional mutant mice (Rbpj ΔM) and control mice. n = 7/9 mice/group, error bars represent s.e.m., *p < 0.05 by two way ANOVA with Bonferroni post test. b Representative flow cytometry of 6 h in vivo BrdU incorporation in macrophages from ischemic muscle at d3. n = 3 independent experiments c Representative flow cytometry of macrophages (CD11b+, F4/80+) from ischemic muscle at d3. n = 4 independent experiments d Gene expression analysis of macrophages sorted from d3 ischemic muscle. n = 3 independent experiments, error bars represent s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05, unpaired Student’s t-test. e Representative cell death analysis in macrophages (CD11b+, F4/80+) from ischemic muscle. n = 4 independent experiments. f Gene expression analysis of isolated Ly6Chi monocytes from wild type or Rbpj ∆M mice (0) and derived macrophages cultured on DLL1 for indicated time. n = 3 independent experiments measured in duplicates, error bars represent s.e.m. ***p < 0.001, **p < 0.01, two-way ANOVA and Bonferroni post-test. g Representative BrdU incorporation of d7 macrophages on DLL1. n = 4 independent experiments
To corroborate the hypothesis that macrophage maturation from Ly6Chi monocytes requires Rbpj we cultured wt or Rbpj-deficient Ly6Chi monocytes on DLL1 and compared proliferation and gene expression patterns in developing macrophages. Mutant Ly6Chi monocytes showed no baseline differences in Etv3, Mafb, Sra and Myc, and readily gave rise to macrophages in culture (Fig. 6f). However, induction of genes involved in macrophage maturation during culture was severly impaired, and the proliferation rate was significantly increased (Fig. 6f, g), thus demonstrating a requirement for Rbpj in macrophage maturation from Ly6Chi monocytes.
Laser Doppler perfusion measurements showed impaired foot perfusion (Fig. 7a) and reduced collateral circulation (Fig. 7b). Histomorphometric studies revealed impaired arteriogenesis in Rbpj mutant mice after HLI, but showed no baseline phenotype (Fig. 7c). This was accompanied by increased ischemic muscle necrosis (Fig. 7d) and impaired muscle regeneration in conditional Rbpj mutant mice (Fig. 7e), which resulted in the development of pronounced muscle fibrosis (Fig. 7f, Supplementary Fig. 17c).
Macrophage Notch signaling regulates arteriogenesis in vivo. a Foot perfusion by laser Doppler perfusion measurement after induction of HLI (0) and during follow up. n = 8/10 mice/group, error bars represent s.e.m. *p < 0.05, ***p < 0.001 vs control by two way ANOVA and Bonferroni post-test. b Representative LDI images of collateral circulation at d3. c Representative H&E images of collateral arteries and quantification of inner circumference, n = 4/6 (ctl), 9/8 (d3), 7/7 (d14) mice/group, error bars represent s.e.m. *p < 0.05, ***p < 0.001 vs control by two way ANOVA and Bonferroni post-test. Scale bar=100 μm. d Overview (×10), magnification (×63) of representative H&E stained muscle sections and quantification of necrosis, n = 5 mice/group, error bars represent s.e.m. ***p < 0.001 vs control by Student’s unpaired t-test. Scale bar=100 μm. e Representative H&E stained muscle sections, quantification of ghost cells and regenerating muscle fibers, n = 6/7 mice/group, error bars represent s.e.m. **p < 0.001, Student’s unpaired t-test. Scale bar=100 μm. f Representative images of Masson-trichrome staining and quantification of fibrosis in muscle sections at d14. n = 6/5 mice/group, error bars represent s.e.m. **p < 0.01, Student’s unpaired t-test. Scale bar=100 μm. g–i Adoptive transfer of monocytes on d1 after HLI in Rbpj ΔM mice. Intravenous injections of PBS (Rbpj ΔM) or 3 × 10e5 BM Ly6Chi monocytes from wild type (+Con) or Rbpj ΔM mice (+Rbpj ΔM). g Foot perfusion by laser Doppler imaging after induction of HLI (0) and during follow up. n = 6/5/5 (d0), 7/5/5 (d3), 6/5/5 (d7), 7/5/5 (d14) mice/group, error bars represent s.e.m. ***p < 0.001, **p < 0.01, *p < 0.05, *comparison between Rbpj ΔM and +Con group, # Comparison between +Con and +Rbpj ΔM groups, Two way ANOVA and Bonferroni post test. h Representative H&E stained images of collateral arteries at d14 and quantification of collateral circumference, n = 7/5/5 mice/group, error bars represent s.e.m. *p < 0.05, ***p < 0.001, one way ANOVA and Bonferroni post-test. Scale bar=100 μm. i Representative images of Masson-trichrome staining and quantification of fibrosis in muscle sections at d14, n = 7/5/5 mice/group, error bars represent s.e.m. *p < 0.05, ***p < 0.001, one way ANOVA and Bonferroni post-test. Scale bar=100 μm
To test whether the ischemic phenotype of Rbpj-deficient mice can be rescued by wild type cells we performed HLI in RbpjΔM mice and intravenously transferred BM Ly6Chi monocytes of either wild type or RbpjΔM mice. Compared with Rbpj conditional mutants with control injections, transfer of wild type monocytes improved the perfusion defects (Fig. 7g), arteriogenesis defect (Fig. 7h) and tissue damage (Fig. 7i) following ischemia, while transfer of Rbpj-deficient monocytes had no discernible effect.
Overall, these data demonstrate that canonical Notch signaling controls macrophage maturation from monocytes and inflammatory polarization, which is required for arteriogenesis and ischemic tissue repair.
Discussion
We here define the molecular and cellular context that regulates the origin and function of macrophages in ischemic neovascularization. Taken together, our results show that blood vessels regulate macrophage differentiation from recruited monocytes, which in turn promotes arteriogenesis and ischemic tissue recovery. Macrophage maturation is controlled by Notch ligand Dll1 expressed in vascular endothelial cells and requires macrophage canonical Notch signaling via Rbpj. Thus, blood vessels foster macrophages for arteriogenesis in response to ischemia.
The origin and maintenance of macrophages after injury can occur either through their differentiation from recruited Ly6Chi monocytes, via local macrophage proliferation or migration of mature macrophages. Using monocyte cell fate tracking and timed elimination studies we show that the transient population of macrophages that arises in ischemic tissues displays a distinct phenotype and develops from an early invading wave of Ly6Chi monocytes by way of maturation. This not only specifies the role of monocytes and macrophages in arteriogenesis4, 5, but also confirms and extends previous reports that Ly6Chi monocytes give rise to macrophages during myocardial ischemia and healing11.
The angiocrine functions of blood vessels, which occur independent of blood flow, are involved in organ maintenance and regeneration12. After injury, instructive cues expressed by local endothelial cells orchestrate the injury response of tissue-resident progenitor cells or fibroblasts during organ regeneration, which in part is mediated by endothelial-specific expression of Notch ligands such as Jag1 13,14,15. We had previously shown that Dll1, which is upregulated in arterial EC upon ischemia, is an essential regulator of arteriogenesis and tissue regeneration20. Our finding that endothelial Dll1 regulates macrophage maturation in vitro and in vivo, which is required for arteriogenesis and ischemic tissue regeneration, extends the spectrum of angiocrine functions towards the setting of ischemic injury, but also demonstrates that angiocrine signaling via Notch ligand Dll1 supports tissue recovery by instructing maturation of recruited cell populations. This is further supported by our finding that transfer of ‘niche’ educated macrophages improves arteriogenesis and tissue repair after HLI. Furthermore, these data also clearly demonstrate a specific, non-redundant role of vascular Dll1 in regulating macrophage maturation, which again emphasizes the ligand-specific nature of Notch signaling events19. Our data also support and expand previous reports on how EC populations cause macrophage polarization28. However, while mature macrophages seem to mediate most of the downstream vascular effects of Dll1, our findings do not necessarily exclude the possibility of Dll1-dependent, intermediary processes occurring within blood vessels involved in the ischemic response.
In addition, our findings also support and extend the concept of on-site education of VEGF-recruited monocytes for arteriogenesis, in which Notch has been implicated35. Altogether, our data clearly demonstrate that angiocrine signaling contributes to neovascularization and tissue recovery via macrophage maturation, thus extending the spectrum of endothelial function. On the other hand, it is possible that dysregulated angiocrine functions are involved in endothelial dysfunction, which is thought to mediate the progression of chronic vascular disease36.
Macrophage diversity is considered to arise from a combination of differentiation programs and activation programs in response to external stimuli6, 9, 10. Our data contribute to the current model in several ways. We demonstrate a requirement for canonical Notch signaling, activated by vascular Dll1, for macrophage differentiation and maturation from Ly6Chi monocytes, thus identifying an angiocrine identity factor for macrophage differentiation. Active Notch signaling promoted a mature phenotype with enhanced phagocytic activity, a low proliferation rate in response to CSF1, improved survival and induction of the terminal differentiation regulator Etv3, thus demonstrating multiple aspects of mature macrophages8. Of note, a related function of Notch in promoting myeloid differentiation has recently been described in myeloid leukemia37. At the same time, macrophage maturation induced by Notch generated cells with high-phagocytic abilities, demonstrated in vitro and suggested by fewer ghost cells in vivo, which also showed an activation profile consistent with tissue-resident or M2-like macrophages7, 38. On the other hand, we also demonstrate that activation/polarization of macrophages and their inflammatory potential is a function of macrophage maturation. Activation of mature macrophages instructed by Notch signaling promoted an anti-inflammatory response under conditions that induce pro-inflammatory cytokines in control macrophages. In addition, the evolution of macrophage maturation over time also dampened the inflammatory response, while re-stimulation with CSF1 induced proliferation and promoted inflammation. Furthermore, the association between macrophage maturation and inflammatory response was also observed in vivo. This demonstrates that maturation critically influences polarization and suggests a modified perspective on macrophage diversity. This hypothesis is also supported by recent findings from systematic gene expression profiling of monocyte to macrophage differentiation, which demonstrated that compared with monocytes, naive macrophages display attenuated innate inflammatory pathways, most likely necessary to generate functional tissue macrophages39.
Our study, in which ligand, cell-source and growth factor context were specifically defined, clearly demonstrates on several levels an anti-inflammatory role of canonical Notch signaling induced by Dll1, which confirms and extends previous reports40, 41. However, other studies show a pro-inflammatory role for Dll4/Notch signaling42,43,44. This suggests that the role of Notch is ligand-, cell-, and context-specific, which again emphasizes the differential effects of specific ligand-receptor combinations19. Nevertheless, together the studies now provide a framework to study the role of Notch signaling components for macrophage phenotypes in specific contexts. Currently, the molecular mediators of Notch in macrophage differentiation and function are unclear. Importantly, loss of Phd2, the enzyme controlling HIF-1α activity, has also been shown to induce macrophage polarization and arteriogenesis5. This would potentially provide a possible mechanism, since Notch and HIF-1α interact under hypoxic conditions in precursor cells during development45. The framework described here can also help to define the factors by which macrophages promote arteriogenesis. Further studies clearly are required to address these issues.
Furthermore, the regulation of cell fate choices of Ly6Chi monocytes are only beginning to emerge. Our cell fate tracking studies of Ly6Chi monocytes during ischemia revealed that, in ischemic muscle, monocytes adopt a macrophage cell fate, but that in other tissues, such as the spleen, cells convert to Ly6Clo monocytes, a process which also requires endothelial Dll121. Thus, while endothelial Dll1 clearly is required for both processes, our findings also suggest that additional, so far unknown, signals ultimately determine cell fate of Ly6Chi monocytes. These signals might constitute tissue-specific factors, or pathology-related milieus, or both.
Last but not least, we here describe a cell culture technique for generation of terminally differentiated macrophages from monocytes under defined conditions ex vivo. These macrophages feature a stable anti-inflammatory phenotype even when challenged with pro-inflammatory stimuli. This provides a setting to study and understand the molecular events driving macrophage maturation and diversity under defined conditions. Moreover, since a recent trial in patients with severe heart failure showed positive results with cell therapy employing an ex vivo expanded cell product enriched for anti-inflammatory ‘M2’ macrophages46, our description might also prove useful to refine cell-therapeutic applications of ex vivo generated macrophages to improve chronic ischemia.
Methods
Mice
Experiments were approved by the State Government (LAVES, Lower Saxony). Mouse strains (Supplementary Table 2) were housed under specific pathogen-free conditions. Around 10–12 week old male mice were used with age- and sex-matched littermate controls.
Mouse strain information
Cx3cr1 GFP/+ mice23, Dll1 +/LacZ mice47, LysM Cre mice34, Rbpj lox/lox mice33, Dll1 lox/lox mice48, Cdh5(PAC)-CreERT2 mice19 have been described. Gt(ROSA)26Sor (Stock number:003309) mice carrying Cre-inducible lacZ alleles were obtained from The Jackson Laboratories and B6.SJL-Ptprc a Pepc b /BoyJ (CD45.1+) (Stock number:002014) mice were obtained from Charles River. The description of the genetic strains used in the study is described in Supplementary Table 2.
Hind limb ischemia
Hind limb Ischemia (HLI), laser Doppler imaging, tissue preparation and analysis of arteriogenesis and muscle sections was carried out as described22 with the following modification: mice were anaesthetized with a mixture of Ketamine (80 mg/kg, Pfizer), Xylazine (2.5 mg/kg, Bayer healthcare), and Midazolam (2.5 mg/kg, Hexal). Briefly, the superficial branch of the left femoral artery was surgically ligated distal to the origin of the deep femoral branch, and growth of pre-existing collateral arteries supplied by the deep femoral branch was studied in semimembranosus muscle sections. Perfusion was measured by Laser Doppler Imaging of plantar regions of interests with Perimed LDPI PIM II Laser Scanner (Perimed, Sweden) and calculated as ratio of left vs. right values. For flow cytometry cell preparation the anterior tibial muscles were anatomically excised and digested in DMEM containing 2 mg/ml collagenase II (type 2, derived from Clostridium histolyticum, Catalog no#LS004176, Worthington Biochemical Corp.) at 37 °C for 20 min using Gentle MACS dissociator (Miltenyi) and filtered through a nylon mesh (40 µm), after which total viable cell number was determined with Trypan blue in a Neubauer chamber.
Animal treatment
Anti-CCR2 mAb (MC21)24 or rat IgG2b control antibody was administered i.p. once a day at a dose of 22.5 µg/mouse, at day 0–2, 0–4, or 4–6 post HLI. Tamoxifen-regulated Cre-recombinase activation was induced as described21 two weeks before experiments. BrdU (1.5 mg, BD Biosciences) was injected i.p. and incorporation was detected after 6 h using BrdU flow kit (BD Biosciences).
Adoptive transfer experiments
Approximately 1–1.5×106 CD11b+GFP+Ly6Chi monocytes were sorted from the bone marrow of CD45.2 Cx3cr1 GFP/+ mice and injected i.v. into CD45.1 mice 1 day after HLI for cell fate tracking studies. For ischemic rescue experiments cells were administered i.v. or through intramuscular injection where indicated. LinnegCD11b+Ly6Chi F4/80/I-Ab/CD11clo/neg splenic monocytes or LinnegCD11b+ F4/80/I-Ab/CD11c+ macrophages (Supplementary Table 1) sorted from d3 ischemic muscle were injected through intramuscular injection into ischemic recipient muscles at d1 after HLI at a dose of 5 × 105 cells in PBS. In a separate rescue experiment 3 × 106 CD11b+GFP+Ly6Chi monocytes were administered through i.v. injection.
Tissue histology and immunohistochemistry
Immunohistochemistry, β-galactosidase, and immunofluorescence staining in mice were performed with modifications from previous descriptions20,21,22. Mice were killed and perfused with 0.1% adenosine (Sigma)/0.05% BSA (Roth)/5000 U heparin (Ratiopharm), followed by fixative 4% PFA (Sigma). Muscles were excised, incubated in 15% (6 h) and 30% (18 h) sucrose and embedded in Tissue-tek OCT compound (Sakura). β-galactosidase staining was performed on PFA fixed slides at 37 °C. Slides were co-stained with anti-mouse CD45 and appropriate alkaline-phosphatase-labeled secondary antibody, then counterstained with eosin, mounted in Vitro-Clud® medium (R. Langenbrinck Labortechnik) and analyzed with Olympus IX71 microscope. For immunofluorescence (IF) and confocal laser scanning microscopy (CLSM) tissue sections were stained using anti-SMA, anti-CD31, and appropriate fluorescence-conjugated secondary antibodies, DAPI and slides were mounted in fluorescence mounting medium (DAKO). Detailed information of the antibodies used can be found in Supplementary Table 5. Whole-mount arterial imaging in limb muscles was performed with a 160 mg/ml pigment particle solution (Schmincke, cat. no. HKS 318) infused via the left ventricle. Images were acquired using Leica TCS SP2 AOBS (Leica Microsystems, Germany) confocal microscope or Zeiss Observer Z1 fluorescence microscope (Zeiss, Germany) respectively.
Flow cytometry
Single-cell suspensions from tissue homogenates or in vitro culture were treated with anti-mouse or anti-human CD16/32 (Trustain-fcX, Biolegend for mouse and FcR Blocking Reagent, Miltenyi Biotec for human) to prevent non-specific binding of antibodies to Fc-receptors. To evaluate apoptosis, cells were stained with 7-AAD and AnnexinV in AnnexinV-binding buffer (all from BioLegend), according to manufacturer’s protocol. After washing, cells were re-suspended in PBS containing 2% FCS, 2 mM Na2EDTA and labeled with primary and secondary antibodies or streptavidin-fluorochrome conjugates (Supplementary Table 5). After staining, cells were washed twice and stained with 7-AAD or Propidium Iodide (Sigma) to exclude dead cells in analysis. The cell suspension was then analyzed on a FACSCalibur or LSR II (BD Biosciences). Sorting was performed on FACSAria (BD Bioscienses) and XDP (Beckman Coulter).
For determination of cell numbers per muscle, the complete anterior tibial muscle was anatomically excised, digested, and total live cell number determined in a Neubauer chamber with trypan blue staining. After flow cytometry, the number of cells per population was determined with the following formula: Trypan blueneg cells×frequency of live/100.
Phagocytosis assay
Macrophages were washed, resuspended in RPMI with 10% FCS, and 105 cells were incubated with 1 μm fluoresbrite red latex beads (Polysciences) at a ratio of 10 beads per cell49. The tubes were incubated at 37 °C or on ice for 1 h, washed and uptake of fluorescent beads was analyzed by flow cytometry.
Quantification of cytokines and chemokines
Approximately 50 µL of cell free supernatants from in vitro cultured macrophages were mixed with 50 µL of the Bioplex resuspension buffer and concentration of cytokines and chemokines was determined using Bio-Plex® Mouse cytokine kit (Biorad) on a Luminex 200 according to manufacturer’s instructions.
Murine cell culture and human co-culture experiments
CD11b+GFP+Ly-6Chi monocytes were sorted from the bone marrow of Cx3cr1 GFP/+ mice and cultured in RPMI1640 supplemented with 10% FCS and either 10 ng/ml recombinant murine CSF1 or CSF2 (Peprotech) based on the experiment. Ligand coating was performed with 1 µg/ml of DLL1-Fc and JAG1-Fc chimeric proteins where indicated (R&D Systems) reconstituted in PBS containing 0.1% BSA for 6 h at room temperature. Recombinant mouse IgG2A-Fc was used as a control. For Notch inhibition assays cells were treated with DAPT (N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester, 3 µM/ml, Sigma Aldrich, Germany), following cell seeding on ligands, and was added to the culture every 24 h. DMSO (Sigma Aldrich, Germany) was added to culture as a control.
Human aortic EC (HAEC, Cat no.: CC-2535), cardiac microvascular EC (MV, Cat no.: CC-7030) were expanded in EGM-2 and EGM-2MV bullet kit medium, as recommended by manufacturer (Clonetics Endothelial cell system, Lonza) and used at P3. Human PB monocytes were isolated with CD14 microbeads (Miltenyi Biotec) and cultured with EC or human M-CSF (Peprotech, 10 ng/ml) in medium supplemented with 1% FCS. For co-culture experiments, EC were seeded at 5000 cells/cm2 and grown for 2 d to form a confluent monolayer. 4 × 104 cells/cm2 CD14+ monocytes were seeded and co-cultured for 24 or 72 h in EBM2 supplemented with 1% FCS+Gentamycin/Amphotericin. Re-isolation after co-culture was performed with CD11b microbeads (Miltenyi Biotec). For estimation of protein levels of CSF1 in co-cultures, 100 µl of the sample aliquots was used for CSF1 estimation in a solid phase sandwich ELISA (96 well format, R&D systems) as per manufacturer’s instructions and the optical density was read at 540 nm. For human CSF1 neutralization experiment, co-cultures were treated with 2 μg/ml anti–CSF1 mAb (Catalog no #MAB216) or isotype mAb (mouse monoclonal IgG2A) from R&D biosciences.
siRNA knockdown experiments
For siRNA studies, cells were transfected with Silencer® Pre-designed siRNA DLL1 (100 nM) (siRNA ID: 133774, Ambion, Applied Biosystems) using GeneTrans II transfection reagent MoBiTec GmbH). Scrambled RNA was used as control. Co-culture of monocytes was established 48 h after transfection.
RNA analysis by real-time quantitative PCR
RNA was isolated with the Nucleospin II kit (Macherey Nagel) and reverse transcription was done using Reverse transcriptase Kit (Invitrogen), according to manufacturer’s protocols. For gene expression analysis, primers were designed using the software Primerquest (IDT) and PrimerBLAST (NCBI) following general guidelines for primer design. Quantitative RT-PCR analysis was performed in duplicates for each sample using Fast start essential green DNA master mix (Roche GmbH) according to manufacturer’s instructions with primers listed in Supplementary Tables 3 and 4 on Light cycler 96 system (Roche). Murine/Human Rps9 was used as a housekeeping gene to normalize expression of gene of interest depending on the experiment. Relative expression was calculated by the comparative CT (2−ΔΔCt values indicate fold change of the gene of interest in the samples relative to a selected control, 2−ΔCt values indicate relative expression of gene of interest between samples normalized to Rps9)50.
Statistical analysis
Results are expressed as mean ± s.e.m. n numbers indicate biological replicates of experiments performed at least three times unless otherwise indicated. Comparison between two groups was calculated using unpaired, two-tailed Student’s t-test or two-tailed paired Student’s t-test with confidence interval of 95% as indicated. For comparison of multiple experimental groups either one-way ANOVA or two-way ANOVA was performed where indicated. Dunnett’s multiple comparision posttest or Bonferroni’s post-test was performed where indicated after performing multiple comparison with ANOVA when the overall p value was < 0.05.
Data availability
The authors declare that all the relevant data are available upon request.
References
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Nahrendorf, M. & Swirski, F. K. Abandoning M1/M2 for a network model of macrophage function. Circ. Res. 119, 414–417 (2016).
Heil, M. & Schaper, W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ. Res. 95, 449–458 (2004).
Arras, M., Ito, W. D., Scholz, D., Winkler, B., Schaper, J. & Schaper, W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J. Clin. Invest. 101, 40–50 (1998).
Takeda, Y. et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479, 122–126 (2011).
Okabe, Y. & Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 17, 9–17 (2016).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Klappacher, G. W. et al. An induced Ets repressor complex regulates growth arrest during terminal macrophage differentiation. Cell 109, 169–180 (2002).
Mantovani, A., Sica, A. & Locati, M. Macrophage polarization comes of age. Immunity 23, 344–346 (2005).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Hilgendorf, I. et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 114, 1611–1622 (2014).
Rafii, S., Butler, J. M. & Ding, B. S. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016).
Butler, J. M. et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264 (2010).
Poulos, M. G. et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 4, 1022–1034 (2013).
Cao, Z. et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat. Med. 22, 154–162 (2016).
Hofmann, J. J. & Iruela-Arispe, M. L. Notch signaling in blood vessels: who is talking to whom about what? Circ. Res. 100, 1556–1568 (2007).
Limbourg, F. P. et al. Essential role of endothelial Notch1 in angiogenesis. Circulation 111, 1826–1832 (2005).
Napp, L. C. et al. Extrinsic notch ligand delta-like 1 regulates tip cell selection and vascular branching morphogenesis. Circ. Res. 110, 530–535 (2012).
Benedito, R. et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124–1135 (2009).
Limbourg, A. et al. Notch ligand Delta-like 1 is essential for postnatal arteriogenesis. Circ. Res. 100, 363–371 (2007).
Gamrekelashvili, J. et al. Regulation of monocyte cell fate by blood vessels mediated by Notch signalling. Nat. Commun. 7, 12597 (2016).
Limbourg, A. et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 4, 1737–1746 (2009).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Bruhl, H. et al. Targeting of Gr-1+,CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum. 56, 2975–2985 (2007).
Gautier, E. L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 (2012).
Satpathy, A. T. et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 209, 1135–1152 (2012).
Clinton, S. K. et al. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am. J. Pathol. 140, 301–316 (1992).
He, H. et al. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood 120, 3152–3162 (2012).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Ziegler-Heitbrock, L. et al. Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80 (2010).
Svensson, J. et al. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 187, 3671–3682 (2011).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 (2002).
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Avraham-Davidi, I. et al. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J. Exp. Med. 210, 2611–2625 (2013).
Vita, J. A. Endothelial function. Circulation 124, e906–e912 (2011).
Lobry, C. et al. Notch pathway activation targets AML-initiating cell homeostasis and differentiation. J. Exp. Med. 210, 301–319 (2013).
Wynn, T. A., Chawla, A. & Pollard, J. W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086–1251086 (2014).
Foldi, J., Shang, Y., Zhao, B., Ivashkiv, L. B. & Hu, X. RBP-J is required for M2 macrophage polarization in response to chitin and mediates expression of a subset of M2 genes. Protein Cell 7, 201–209 (2016).
Zhang, Q. et al. Notch signal suppresses Toll-like receptor-triggered inflammatory responses in macrophages by inhibiting extracellular signal-regulated kinase 1/2-mediated nuclear factor kappaB activation. J. Biol. Chem. 287, 6208–6217 (2012).
Xu, H. et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 13, 642–650 (2012).
Wang, Y. C. et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 70, 4840–4849 (2010).
Fukuda, D. et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl Acad. Sci. USA 109, E1868–1877 (2012).
Gustafsson, M. V. et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9, 617–628 (2005).
Patel, A. N. et al. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet 387, 2412–2421 (2016).
Hrabe de Angelis, M., McIntyre, J. 2nd & Gossler, A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 386, 717–721 (1997).
Hozumi, K. et al. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat. Immunol. 5, 638–644 (2004).
Bhatnagar, N. et al. Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood 116, 1308–1316 (2010).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Acknowledgements
We thank S.H., M.M. and S.S. for excellent technical assistance; the Research Core Facilities Cell Sorting and Laser Microscopy for support. This research has been funded by grants from Deutsche Forschungsgemeinschaft (Li948/5-1), Bundesministerium für Bildung und Forschung (01GU1105B, IFB-Tx 01EO1302), and German-Israeli-Foundation (1089) to F.P.L.
Author information
Authors and Affiliations
Contributions
K.K., A.L., T.K., J.G., C.B., C.H., C. F., V. J. L., L.C.N. did experiments; K.K., A.L., T.K., J.G., C.F., L.C.N., F.P.L. analysed experiments; K.K., A.L., T.K., J.G., C.F., M.M., C.W., F.P.L. designed experiments; K.K., A.L., T.K., J.B., H.H., C.W., R.H.A., F.P.L. wrote and edited the manuscript; F.P.L. conceived and directed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krishnasamy, K., Limbourg, A., Kapanadze, T. et al. Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat Commun 8, 952 (2017). https://doi.org/10.1038/s41467-017-00953-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-017-00953-2
This article is cited by
-
A new fasciocutaneous flap model identifies a critical role for endothelial Notch signaling in wound healing and flap survival
Scientific Reports (2023)
-
Sourcing of human peripheral blood-derived myeloid angiogenic cells under xeno-free conditions for the treatment of critical limb ischemia
Stem Cell Research & Therapy (2022)
-
C-type lectin receptor CLEC4A2 promotes tissue adaptation of macrophages and protects against atherosclerosis
Nature Communications (2022)
-
Brain milieu induces early microglial maturation through the BAX-Notch axis
Nature Communications (2022)
-
Loss of vascular endothelial notch signaling promotes spontaneous formation of tertiary lymphoid structures
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.