Abstract
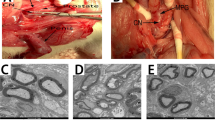
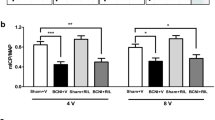
To determine if the insulin-like growth factor-1 (IGF-1) pathway is involved in the improvement in erectile function recovery in rats after nerve crush injury treated with pioglitazone (Pio). Sprague-Dawley rats were divided into four groups. The first group received sham operation (n = 5). The second group underwent bilateral cavernous nerve injury (BCNI, n = 7). The third group received BCNI and Pio treatment (BCNI + Pio, n = 7), whereas the fourth group underwent BCNI with Pio treatment and IGF-1 inhibition (BCNI + Pio + JB-1, n = 7). The IGF-1 receptor (IGF-1R) was inhibited by JB-1, a small molecular antagonist of the receptor. After 14 days of treatment, erectile function was measured via intracorporal pressure normalized to mean arterial pressure (ICP/MAP) and the major pelvic ganglion and cavernous nerve harvested for western blot and immunohistochemistry (IHC) of phosphorylated-IGF-1Rβ (p-IGF-1Rβ), phosphorylated-ERK1/2 (p-ERK1/2), and neuronal NOS (nNOS). BCNI + Pio animals exhibited improvements in ICP/MAP, similar to Sham animals, and BCNI + Pio + JB-1 rats demonstrated a reduced ICP/MAP similar to BCNI-only rats at all measured voltages. Western blot results showed upregulation of p-IGF-1Rβ was observed in the BCNI + Pio group. Low levels of p-ERK1/2 were seen in the JB-1-treated animals. The immunoblot results were supported by IHC findings. Intense IHC staining of nNOS was detected in the BCNI + Pio group. The group treated with JB-1 showed minimal protein expression of p-ERK1/2, nNOS, and p-IGF-1Rβ. Pio improves erectile function in rats undergoing BCNI via an IGF-1-mediated pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3:87–92.
Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 2016;197:S165–70.
Schauer I, Keller E, Muller A, Madersbacher S. Have rates of erectile dysfunction improved within the past 17 years after radical prostatectomy? A systematic analysis of the control arms of prospective randomized trials on penile rehabilitation. Andrology. 2015;3:661–5.
Weyne E, Mulhall J, Albersen M. Molecular pathophysiology of cavernous nerve injury and identification of strategies for nerve function recovery after radical prostatectomy. Curr Drug Targets. 2015;16:459–73.
Chung E, De Young L, Brock GB. Investigative models in erectile dysfunction: a state-of-the-art review of current animal models. J Sex Med. 2011;8:3291–305.
Quinlan DM, Nelson RJ, Partin AW, Mostwin JL, Walsh PC. The rat as a model for the study of penile erection. J Urol. 1989;141:656–61.
Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–96.
Aliperti LA, Lasker GF, Hagan SS, Hellstrom JA, Gokce A, Trost LW et al. Efficacy of pioglitazone on erectile function recovery in a rat model of cavernous nerve injury. Urology. 2014;84:1122–7.
Katz EG, Moustafa AA, Heidenberg D, Haney N, Peak T, Lasker GF, et al. Pioglitazone enhances survival and regeneration of pelvic ganglion neurons after cavernosal nerve injury. Urology. 2016;89:76–82.
Smith U. Pioglitazone: mechanism of action. Int J Clin Pract Suppl. 2001;121:13–8.
Kintscher U, Law RE. PPARgamma-mediated insulin sensitization: the importance of fat versus muscle. Am J Physiol Endocrinol Metab. 2005;288:E287–91.
Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–88.
Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448:121–38.
Higashi Y, Holder K, Delafontaine P. Thiazolidinediones upregulate insulin-like growth factor-1 receptor via a peroxisome proliferator-activated receptor gamma-independent pathway. J Biol Chem. 2010;285:36361–8.
Mughal A, Kumar D, Vikram A. Effects of thiazolidinediones on metabolism and cancer: relative influence of PPARgamma and IGF-1 signaling. Eur J Pharmacol. 2015;768:217–25.
Bochinski D, Hsieh PS, Nunes L, Lin GT, Lin CS, Spencer EM, et al. Effect of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 complex in cavernous nerve cryoablation. Int J Impot Res. 2004;16:418–23.
Sjoberg J, Kanje M. Insulin-like growth factor (IGF-1) as a stimulator of regeneration in the freeze-injured rat sciatic nerve. Brain Res. 1989;485:102–8.
Apel PJ, Ma J, Callahan M, Northam CN, Alton TB, Sonntag WE, et al. Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve. 2010;41:335–41.
Sumino Y, Yoshikawa S, Mori K, Mimata H, Yoshimura N. IGF-1 as an important endogenous growth factor for recovery from impaired urethral continence function in rats with simulated childbirth injury. J Urol. 2016;195:1927–35.
Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314:265–77.
Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–81.
Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiol Drug Saf. 2007;16:485–92.
Boxall N, Bennett D, Hunger M, Dolin P, Thompson PL. Evaluation of exposure to pioglitazone and risk of prostate cancer: a nested case-control study. BMJ Open Diabetes Res Care. 2016;4:e000303.
Suzuki S, Mori Y, Nagano A, Naiki-Ito A, Kato H, Nagayasu Y, et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, suppresses rat prostate carcinogenesis. Int J Mol Sci. 2016;17:2027.
Erdmann E, Harding S, Lam H, Perez A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes Metab. 2016;18:266–73.
Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139.
Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305.
Chattopadhyay S, Shubayev VI. MMP-9 controls Schwann cell proliferation and phenotypic remodeling via IGF-1 and ErbB receptor-mediated activation of MEK/ERK pathway. Glia. 2009;57:1316–25.
Rothe F, Langnaese K, Wolf G. New aspects of the location of neuronal nitric oxide synthase in the skeletal muscle: a light and electron microscopic study. Nitric Oxide. 2005;13:21–35.
Carnicer R, Suffredini S, Liu X, Reilly S, Simon JN, Surdo NC, et al. The subcellular localization of neuronal nitric oxide synthase determines the downstream effects of NO on myocardial function. Cardiovasc Res. 2017;113:321–31.
Funding
Funding for this project was provided by the Sexual Medicine Society of North America.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Heidenberg, D., Haney, N., Rezk, B.M. et al. Pioglitazone’s beneficial effects on erectile function preservation after cavernosal nerve injury in the rat are negated by inhibition of the insulin-like growth factor-1 receptor: a preclinical study. Int J Impot Res 31, 1–8 (2019). https://doi.org/10.1038/s41443-018-0054-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-018-0054-2
This article is cited by
-
A sugar sweet new treatment for erectile dysfunction after radical prostatectomy
International Journal of Impotence Research (2019)