Abstract
The complement system has recently been reported to contribute to the development and pathogenesis of hypertension, several cardiovascular and renal diseases, and cardiometabolic disorders accompanied by inflammation and tissue remodeling. We have demonstrated that complement 3 (C3) is highly expressed in mesenchymal tissues in spontaneously hypertensive rats (SHRs) and induces the synthetic phenotype and exaggerated growth of mesenchymal cells by maintenance effect on dedifferentiated cells. To verify the role of C3 in the pathogenesis of hypertension, we targeted the C3 gene from SHRs by zinc-finger nuclease gene-editing technology and demonstrated that the increased expression of C3 induces salt-sensitive hypertension with activation of the renal renin-angiotensin system in SHRs. We recently found that increased expression of C3 is associated with the suppression of miR145 and induces Krüppel-like factor 5 and the synthetic phenotype of mesenchymal cells in SHRs. We also demonstrated that C3 is involved in the epithelial-to-mesenchymal transition and dedifferentiation of epithelial cells in kidneys subjected to unilateral ureteral obstruction with elevation of blood pressure. Thus, C3 is an essential factor in the pathogenesis of hypertension due to its maintenance effect on undifferentiated mesenchymal cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
de Bruijn MH, Fey GH. Human complement component C3. cDNA coding sequence and derived primary structure. Proc Natl Acad Sci USA. 1985;82:708–12.
Legoedec J, Gasque P, Jeanne JF, Fontaine M. Expression of the complement alternative pathway by human myoblasts in vitro: biosynthesis of C3, factor B, factor H and factor I. Eur J Immunol. 1995;25:3460–6.
Lévi-Strauss M, Mallat M. Primary cultures of murine astrocytes produce C3 and factor B, two components of the alternative pathway of complement activation. J Immunol. 1987;139:2361–6.
Schumacher WA, Fantone JC, Kunkel SE, Webb RC, Lucchesi BR. The anaphylatoxins C3a and C5a are vasodilators in the canine coronary vasculature in vitro and in vivo. Agents Actions. 1991;34:345–9.
Schraufstatter IU, Trieu K, Sikora L, Sriramarao P, DiScipio R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002;169:2102–10.
Usami M, Mitsunaga K, Miyajima A, Sunouchi M, Doi O. Complement component C3 functions as an embryotrophic factor in early postimplantation rat embryos. Int J Dev Biol. 2010;54:1277–85.
Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182:3827–36.
Sarafidis PA, Georgianos P, Bakris GL. Resistant hypertension—its identification and epidemiology. Nat Rev Nephrol. 2013;9:51–58.
Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, et al. Hypertension: physiology and pathophysiology. Compr Physiol. 2012;2:2393–442.
Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621–35.
Cuhlmann S, Van der Heiden K, Saliba D, Tremoleda JL, Khalil M, Zakkar M, et al. Disturbed blood flow induces RelA expression via c-Jun N-terminal kinase 1: a novel mode of NF-kappaB regulation that promotes arterial inflammation. Circ Res. 2011;108:950–9.
Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–63.
Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Investig. 2007;117:568–75.
Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56.
Franco M, Tapia E, Santamaría J, Zafra I, García-Torres R, Gordon KL, et al. Renal cortical vasoconstriction contributes to development of salt-sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol. 2001;12:2263–71.
Sánchez-Lozada LG, Tapia E, Johnson RJ, Rodríguez-Iturbe B, Herrera-Acosta J. Glomerular hemodynamic changes associated with arteriolar lesions and tubulointerstitial inflammation. Kidney Int. 2003;64:S9–S14.
Sumida T, Naito AT, Nomura S, Nakagawa A, Higo T, Hashimoto A, et al. Complement C1q-induced activation of β-catenin signalling causes hypertensive arterial remodelling. Nat Commun. 2015;6:6241.
Cove-Smith A, Hendry BM. The regulation of mesangial cell proliferation. Nephron Exp Nephrol. 2008;108:e74–9.
Abboud HE. Mesangial cell biology. Exp Cell Res. 2012;318:979–85.
Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC 3rd. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 2008;57:1439–45.
Johnson RJ, Raines EW, Floege J, Yoshimura A, Pritzl P, Alpers C, et al. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992;175:1413–6.
Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep. Biol Med. 1967;25:257–64.
Svendsen UG. Influence of neonatal thymectomy on blood pressure and hypertensive vascular disease in rats with renal hypertension. Acta Pathol Microbiol Scand A. 1975;83:199–205.
Olsen F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol Microbiol Scand C. 1980;88:1–5.
Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–37.
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60.
Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ. 2014;38:20–24.
Rudemiller NP, Crowley SD. Interactions between the immune and the renin-angiotensin systems in hypertension. Hypertension. 2016;68:289–96.
Laufer J, Katz Y, Paswell J. Extrahepatic synthesis of complement proteins in inflammation. Mol Immunol. 2001;38:221–9.
Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013;25:47–53.
Hertle E, Stehouwer CD, van Greevenbroek MM. The complement system in human cardiometabolic disease. Mol Immunol. 2014;61:135–48.
Li W, Tada T, Miwa T, Okada N, Ito J, Okada H, et al. mRNA expression of complement components and regulators in rat arterial smooth muscle cells. Microbiol Immunol. 1999;43:585–93.
Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev. 2001;180:35–48.
Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3–the “Swiss Army Knife” of innate immunity and host defense. Immunol Rev. 2016;274:33–58.
Oksjoki R, Kovanen PT, Pentikäinen MO. Role of complement activation in atherosclerosis. Curr Opin Lipido. 2003;14:477–82.
Shagdarsuren E, Wellner M, Braesen JH, Park JK, Fiebeler A, Henke N, et al. Complement activation in angiotensin II-induced organ damage. Circ Res. 2005;97:716–24.
Welch TR. The complement system in renal diseases. Nephron. 2001;88:199–204.
Onat A, Can G, Rezvani R, Cianflone K. Complement C3 and cleavage products in cardiometabolic risk. Clin Chim Acta. 2011;412:1171–9.
Zhang C, Li Y, Wang C, Wu Y, Cui W, Miwa T, et al. Complement 5a receptor mediates angiotensin II-induced cardiac inflammation and remodeling. Arterioscler Thromb Vasc Biol. 2014;34:1240–8.
Bao X, Meng G, Zhang Q, Liu L, Wu H, Du H, et al. Elevated serum complement C3 levels are associated with prehypertension in an adult population. Clin Exp Hypertens. 2017;39:42–49.
Engström G, Hedblad B, Berglund G, Janzon L, Lindgärde F. Plasma levels of complement C3 is associated with development of hypertension: a longitudinal cohort study. J Hum Hypertens. 2007;21:276–82.
Seifert PS, Hugo F, Hansson GK, Bhakdi S. Prelesional complement activation in experimental atherosclerosis. Terminal C5b-9 complement deposition coincides with cholesterol accumulation in the aortic intima of hypercholesterolemic rabbits. Lab Investig. 1989;60:747–54.
Buono C, Come CE, Witztum JL, Maguire GF, Connelly PW, Carroll M, et al. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–31.
Hsu SI, Couser WG. Chronic progression of tubulointerstitial damage in proteinuric renal disease is mediated by complement activation: a therapeutic role for complement inhibitors? J Am Soc Nephrol. 2003;14:S186–S191.
Sen S, Tarazi RC, Khairallah PA, Bumpus FM. Cardiac hypertrophy in spontaneously hypertensive rats. Circ Res. 1974;35:775–81.
Walter SV, Hamet P. Enhanced DNA synthesis in heart and kidney of newborn spontaneously hypertensive rats. Hypertension. 1986;8:520–5.
Hu WY, Fukuda N, Kanmatsuse K. Growth characteristics, angiotensin II-generation, and microarray-determined gene expression in vascular smooth muscle cells from young spontaneously hypertensive rats. J Hypertens. 2002;20:1323–33.
Fukuda N, Satoh C, Hu WY, Soma M, Kubo A, Kishioka H, et al. Production of angiotensin II by homogeneous cultures of vascular smooth muscle cells from spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1999;19:1210–7.
Fukuda N, Hu WY, Satoh C, Nakayama M, Kishioka H, Kubo A, et al. Contribution of synthetic phenotype on the enhanced angiotensin II-generating system in vascular smooth muscle cells from spontaneously hypertensive rats. J Hypertens. 1999;17:1099–107.
Hu WY, Fukuda N, Satoh C, Jian T, Kubo A, Nakayama M, et al. Phenotypic modulation by fibronectin enhances the angiotensin II-generating system in cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:1500–5.
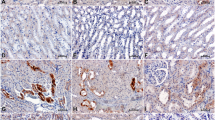
Lin ZH, Fukuda N, Jin XQ, Yao EH, Ueno T, Endo M, et al. Complement 3 is involved in the synthetic phenotype and exaggerated growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2004;44:42–47.
Ikeda K, Fukuda N, Ueno T, Endo M, Kobayashi N, Soma M, et al. Role of complement 3a in the growth of mesangial cells from stroke-prone spontaneously hypertensive rats. Clin Exp Hypertens. 2014;36:58–63.
Negishi E, Fukuda N, Otsuki T, Katakawa M, Komatsu K, Chen L, et al. Involvement of complement 3 in the salt-sensitive hypertension by activation of renal renin-angiotensin system in spontaneously hypertensive rats. Am J Physiol Ren Physiol. 2018;315:F1747–F1758.
Chen L, Fukuda N, Otsuki T, Tanaka S, Nakamura Y, Kobayashi H, et al. Increased complement 3 with suppression of miR-145 induces the synthetic phenotype in vascular smooth muscle cells from spontaneously hypertensive rats. J Am Heart Assoc. 2019;8:e012327.
Wan JX, Fukuda N, Endo M, Tahira Y, Yao EH, Matsuda H, et al. Complement 3 is involved in changing the phenotype of human glomerular mesangial cells. J Cell Physiol. 2007;213:495–501.
Yao EH, Fukuda N, Ueno T, Tsunemi A, Endo M, Matsumoto K. Complement 3 activates KLF5 gene in vascular smooth muscle cells. Biochem Biophys Res Commun. 2008;367:468–73.
Han Y, Fukuda N, Ueno T, Endo M, Ikeda K, Xueli Z, et al. Role of complement 3a in the synthetic phenotype and angiotensin II-production in vascular smooth muscle cells from spontaneously hypertensive rats. Am J Hypertens. 2012;25:284–9.
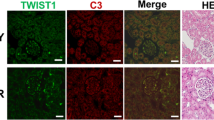
Cho MS, Rupaimoole R, Coi HJ, Noh K, Chen J, Hu Q, et al. Complement component 3 (C3) is regulated by TWIST1 and mediates epithelial-mesenchymal transition (EMT). J Immunol. 2016;196:1412–8.
Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol. 2003;23:1510–20.
Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801.
Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–64.
Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev. 2017;97:1555–617.
Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–8.
Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95:194–204.
Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, et al. Wnt4/beta-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–436.
Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–66.
Verdeguer F, Castro C, Kubicek M, Pla D, Vila-Caballer M, Vinué A, et al. Complement regulation in murine and human hypercholesterolemia and role in the control of macrophage and smooth muscle cell proliferation. Cardiovasc Res. 2007;76:340–50.
Chen L, Fukuda N, Shimizu S, Kobayashi H, Tanaka H, Nakamura Y, et al. Role of complement 3 in renin generation during the differentiation of mesenchymal stem cells to smooth muscle cells (under submission).
Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD, et al. Perivascular adipose tissue-derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone. Arterioscler Thromb Vasc Biol. 2010;30:2568–74.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210.
Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–28.
Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, et al. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Dev Biol. 2001;240:404–18.
Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, et al. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001;159:1313–21.
Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, et al. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62:137–46.
Skromne I, Stern CD. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development. 2001;128:2915–27.
Feng Q, Di R, Tao F, Chang Z, Lu S, Fan W, et al. PDK1 regulates vascular remodeling and promotes epithelial-mesenchymal transition in cardiac development. Mol Cell Biol. 2010;30:3711–21.
Banerjee I, Zhang J, Moore-Morris T, Lange S, Shen T, Dalton ND, et al. Thymosin Beta 4 is dispensable for murine cardiac development and function. Circ Res. 2012;110:456–64.
Wan J, Zhou X, Cui J, Zou Z, Xu Y, You D. Role of complement 3 in TNF-α-induced mesenchymal transition of renal tubular epithelial cells in vitro. Mol Biotechnol. 2013;54:92–100.
Suetsugu-Maki R, Maki N, Fox TP, Nakamura K, Cowper Solari R, Tomlinson CR, et al. A complement receptor C5a antagonist regulates epithelial to mesenchymal transition and crystallin expression after lens cataract surgery in mice. Mol Vis. 2011;17:949–64.
Liu F, Gou R, Huang J, Fu P, Chen F, Fan WX, et al. Effect of anaphylatoxin C3a, C5a on the tubular epithelial-myofibroblast transdifferentiation in vitro. Chin Med J. 2011;124:4039–45.
Tang Z, Bao L, Hatch E, Sacks SH, Sheerin NS. C3a mediates epithelial-to-mesenchymal transition in proteinuric nephropathy. J Am Soc Nephrol. 2009;20:593–603.
Zhou X, Fukuda N, Matsuda H, Endo M, Wang X, Saito K, et al. Complement 3 activates the renal renin-angiotensin system by induction of epithelial-to-mesenchymal transition of the nephrotubulus in mice. Am J Physiol Ren Physiol. 2013;305:F957–F967.
Norman JT, Fine LG. Progressive renal disease: fibroblasts, extracellular matrix, and integrins. Exp Nephrol. 1999;7:167–77.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, L., Fukuda, N., Matsumoto, T. et al. Role of complement 3 in the pathogenesis of hypertension. Hypertens Res 43, 255–262 (2020). https://doi.org/10.1038/s41440-019-0371-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0371-y
Keywords
This article is cited by
-
Beneficial effects of Panax notoginseng (Burkill) F. H. Chen flower saponins in rats with metabolic hypertension by inhibiting the activation of the renin–angiotensin–aldosterone system through complement 3
BMC Complementary Medicine and Therapies (2023)
-
TWIST1 transcriptionally upregulates complement 3 in glomerular mesangial cells from spontaneously hypertensive rats
Hypertension Research (2022)
-
Increased expression of acyl-CoA oxidase 2 in the kidney with plasma phytanic acid and altered gut microbiota in spontaneously hypertensive rats
Hypertension Research (2021)