Abstract
Nocturia in older adults has been reported to be a risk factor for cardiovascular outcomes, and the stiffening of large arteries might be an underlying mechanism. To clarify the possible association between nocturia and arterial stiffness, we analyzed a dataset from the Japanese general population. Study participants consisted of 5928 community residents (mean age: 60.0 ± 11.8 years). The frequency of nocturnal urination was recorded for 1 week using a sleep diary. Arterial stiffness was assessed by brachial-to-ankle pulse wave velocity (baPWV). Sleep blood pressure was measured automatically at 0000, 0200, and 0400 hours by wearing a cuff on the upper arm during sleep. The mean baPWV was 1278 ± 227 cm/s. The frequency of nocturnal urination showed a linear positive association with baPWV (P < 0.001). The association between a sleep diary-based nocturnal urination frequency > 1.5 times/night (corresponding to a ≥ 2 times/night frequency obtained by the questionnaire) and baPWV remained significant after adjusting for major covariates, including office blood pressure (β = 0.051, P < 0.001) and sleep blood pressure (β = 0.040, P < 0.001). This association was more prominent in men (β = 0.069, P < 0.001) than in women (β = 0.023, P = 0.013), particularly in older (β = 0.068, P = 0.006) compared with younger (β = 0.029, P = 0.270) men. Frequent nocturnal urination was independently associated with baPWV in older men. Nocturia may be a marker for cardiovascular disease risks that cannot be assessed by conventional risk factors such as blood pressure.
Similar content being viewed by others
Introduction
Nocturia is a frequently observed phenomenon, particularly in older individuals [1]. Nocturia has been reported to be a major cause of poor self-reported health and is strongly associated with depressive and anxiety symptoms [2]. Although a major cause of nocturia has been considered to be polyuria, particularly nocturnal polyuria [1], systemic atherosclerosis has also been suggested as another causal factor of nocturia. Atherosclerosis may decrease bladder compliance and cause lower urinary tract syndromes, including nocturia, by decreasing blood perfusion at the bladder neck [3]. Given findings supporting the concept of nocturia as a risk factor for increased coronary heart disease and mortality [4], it was hypothesized that this urinary condition may represent potential systemic atherosclerosis.
We have previously reported that nocturia is strongly associated with high nocturnal blood pressure (BP), independent of poor sleep characteristics, and that individuals with frequent urination show smaller nocturnal BP dips [5]. Because nighttime BP is a stronger predictor of composite cardiovascular endpoints than daytime BP [6] and abnormalities in circadian BP rhythm have been reported to be a risk factor for atherosclerotic vascular changes [7], there may be intercorrelations between nocturia and structural changes in the large artery that are mediated by high BP during sleep.
To clarify whether nocturia represents a structural change in the large artery independent of BP during sleep, we analyzed a dataset from the Nagahama study, a general population-based cohort study conducted in Japan, for which the number of nocturnal urinations, BP values at home and while sleeping, and arterial stiffness measurements were available for analysis.
Methods
Study participants
In this cross-sectional study, we analyzed a dataset of the second-visit investigation of the Nagahama study, performed between 2013 and 2016 (n = 9.850) [8, 9]. Participants in this study are community residents from Nagahama, a rural city of 125,000 inhabitants located in central Japan. Individuals aged between 30 and 74 years old during recruitment, living independently without physical impairment or dysfunction, were eligible to participate.
Among a total of 6249 participants who successfully measured BP at home and while sleeping, which was an optional examination provided upon request, 5928 participants were ultimately included in the analysis after applying the following exclusion criteria: pregnancy (n = 4), pacemaker implantation (n = 4), hemodialysis therapy (n = 4) obstructive sleep apnea therapy (n = 33), suspected cases of shift working (n = 12), severe renal functional decline [estimated glomerular filtration rate < 30 ml/min/1.73 m2 or urinary albumin ≥ 300 mg/day] (n = 40), large bilateral differences in brachial-to-ankle pulse wave velocity (baPWV) (n = 34), lack of clinical data or incomplete response to a questionnaire used in this study (n = 1), and lack of a sleep diary (n = 189).
All study procedures were approved by the Ethics Committee of Kyoto University Graduate School of Medicine and by the Nagahama Municipal Review Board. Written informed consent was obtained from all participants.
Frequency of nocturnal urination
The frequency of nocturnal urination each night was recorded by the participants in their sleep diary for 7 days. Means of all recorded values were used for analysis. Subjective frequency of nocturnal urination was assessed using the International Prostate Symptom Score, which considers one of the score’s 7 items as relevant for a usual frequency of nocturnal urination.
Arterial stiffness assessment
Arterial stiffness was assessed by baPWV. To measure baPWV, cuffs were attached to the brachia and ankles, and pulse volume waveforms were simultaneously recorded while the participant was in a sitting position, using a plethysmographic sensor connected to the cuffs (Vasera-1500, Fukuda Denshi Co., Ltd., Tokyo, Japan). baPWV was calculated from the time interval between the wave fronts of the brachial and ankle waveforms and the path length from the brachia to the ankle calculated from the participant’s body height [10]. baPWV has been reported to be closely correlated with carotid–femoral PWV, a standard measure of arterial stiffness [11, 12].
Home BP measurement
Participants were required to measure home BP (morning and evening BP) for 7 days and sleep BP for the last 5 nights (days 3 to 7) using an automatic cuff-oscillometric device (HEM-7080IC, Omron Healthcare, Kyoto, Japan) [5] according to the following procedures described in the guidelines from the Japanese Society of Hypertension [13]; (1) morning BP: measure BP in a sitting position within 1 h of waking up, after urination and a few minutes of rest in a sitting position, and before taking antihypertensive drugs and eating breakfast; (2) evening BP: measure BP in a sitting position within 1 h before going to bed after urination and a few minutes of rest in a sitting position; and (3) sleep BP: measure BP during sleep by positioning the cuff on the upper arm before going to sleep. The BP monitor was programmed to automatically measure BP at 0000, 0200, and 0400 hours. All BP values were stored in the built-in memory of the device.
Among the different recorded BP values, including irregularly measured BP values, sleep BP was determined with reference to actigraphy, recorded using a wrist-wearable activity monitor (Actiwatch 2 or Actiwatch Spectrum Plus, Philips Respironics, Murrysville, PA, USA), and a sleep diary. BP values measured within 1 h after waking up and within 1 h before sleeping, also determined with reference to actigraphy, were considered morning and evening BPs, respectively. If there were multiple readings in each 1-h slot, the mean value was calculated as the representative value. Participants with continuous evening, sleep, and morning BP measurements for at least 1 day were included in the analysis. The mean value of all measurements was used for analysis.
Basic clinical parameters
The basic clinical parameters used in this study were obtained from personal records measured at the second-visit investigation.
Office BP was measured at the second-visit investigation using a standard cuff-oscillometric device (HEM-9000AI, Omron Healthcare, Kyoto, Japan). Measurements were taken twice after a few minutes of rest in a sitting position, and the mean value of both measurements was used for the analysis. Hypertension was defined as systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, and/or the use of antihypertensive drugs.
Smoking and alcohol drinking habits and medication use were queried using a structured questionnaire. Alcohol consumption was calculated by multiplying the amount of alcohol consumed in a single day by the number of drinking days per week and is represented using traditional Japanese units of alcohol (Go), where 1 Go corresponds to 22 g of ethanol. Diabetes was defined as high glucose levels [fasting ( ≥ 4 h): ≥ 126 mg/dl; occasional: ≥ 200 mg/dl], HbA1c ≥ 6.5%, and/or use of antihyperglycemic drugs.
Statistical analysis
Data are expressed as the mean ± standard deviation or frequency. Group differences in continuous and categorical variables were assessed by analysis of variance and chi-squared tests, respectively. Linear regression analysis was used to identify factors independently associated with baPWV. Statistical analyses were performed using JMP Pro 14.2.0 software (SAS Institute Inc., Cary, NC, USA). A P value < 0.05 was considered statistically significant.
Results
The clinical characteristics of the study participants are summarized in Table 1. In the total population, a sleep diary was available for 6.6 ± 1.1 days. Figure 1 shows the association between nocturnal urination frequency assessed using the sleep diary and the self-administered questionnaire. Because frequencies were considerably different due to measurement methods, the usual nocturia definition (≥1-time voiding/night), as well as the clinically relevant nocturnal voiding frequency (≥2 time/night), according to a questionnaire, were not simply extrapolated in this analysis.
Correlation between nocturnal urination frequency determined through a sleep diary and a questionnaire. Sleep diary-based frequency was calculated as the mean of all values recorded in a 7-day sleep diary. Questionnaire-based frequency was obtained using the International Prostate Symptom Score (IPSS)
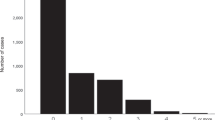
The frequency of nocturnal urination showed a linear positive association with baPWV, both in the sleep diary-based analysis (Fig. 2) and in the questionnaire-based analysis (none: 1197 ± 189, 1 time: 1299 ± 233, 2 times: 1407 ± 233, 3 times: 1453 ± 220, ≥ 4 times: 1394 ± 259 cm/s, P < 0.001). Although other factors, including age, sex, systolic BP, and body mass index (BMI), were also significantly associated with the frequency of nocturnal urination (all P < 0.001), the association of nocturnal urination – particularly >1.5 times/night – with baPWV remained significant after adjusting for these covariates, as did current smoking, alcohol consumption, use of antihypertensive drugs, HbA1c, triglycerides, and high-density lipoprotein cholesterol (Table 2). The association between frequent nocturnal urination (>1.5 times/night) and baPWV (Table 3, Model 1) was independent of sleep systolic BP (Model 2, VIF = 1.93) and was more prominent in men (Model 3) than women (Model 6), particularly in older (Model 4) compared with younger (Model 5) men; this finding was possibly due to the higher frequency of nocturnal urination in the older population (Fig. 3).
Differences in baPWV according to frequency of nocturnal urination. Values represent the mean baPWV. Frequency of nocturnal urination was determined using a sleep diary. Statistical significance was assessed by analysis of variance. The number of participants in each subgroup is shown in the columns
Discussion
In this cross-sectional study conducted in a large general population, we found a strong positive association between the frequency of nocturnal urination and arterial stiffness in older men. Sleep diary-assessed nocturnal urination frequency >1.5 times/night (which is close to the generally accepted clinically relevant frequency of ≥2 times/night obtained by conventional questionnaire method) was identified as a risk factor for high baPWV. This association was independent of sleep BP and more prominent in men, particularly older men. Nocturia may therefore be a useful marker for potential cardiovascular disease risk that cannot be assessed by conventional risk factors, such as high BP.
According to a previous report from our group, the frequency of nocturnal urination is a strong determinant of increased sleep BP [5]. Although high BP, either an office- or home-measured value, is a strong risk factor for arterial stiffness, the association between nocturnal urination frequency and baPWV was independent of BP, suggesting a BP-independent pathophysiological relationship between nocturnal urination and arterial stiffness. Although the cross-sectional observational nature of this study did not allow us to clarify its underlying mechanisms, bladder ischemia caused by arterial stiffness that worsens voiding symptoms via decreased bladder compliance may be hypothesized as a plausible explanation. An observational study [3] found a significant decrease in blood perfusion of the bladder neck in patients with lower urinary tract symptoms and that blood perfusion level was inversely associated with nocturnal urination frequency.
Nocturia may thus be a consequence but not a cause of arterial stiffness. However, as nocturnal urination frequency is easily assessable and accurately measurable by multiday recording, frequent nocturnal urination should be regarded as a simple marker for potential arterial stiffness. We investigated the nocturnal urination frequency using a sleep diary, and the frequencies were considerably different from those observed using a self-administered questionnaire. When the frequency was assessed by the conventional questionnaire, a ≥ 2 times/night frequency may therefore be the threshold considered for risk of arterial stiffness.
The association between nocturnal urination and baPWV was prominent in older men, possibly due to the higher urination frequency in this subgroup. However, there was a nonnegligible number of younger men and women complaining of frequent urination during the night. Additional large-scale studies may clarify whether urination frequency in the middle-aged population should be overlooked as a potential risk factor for arterial stiffness.
The association between nocturia and arterial stiffness was somewhat weakened by adjustment for sleep BP, suggesting that the association was partially mediated by high sleep BP. High BP, measured either awake or sleeping, is a well-known risk factor for arterial stiffness, and sleep BP lowering intervention may be effective, particularly in individuals with nocturia, not only to decrease urination frequency but also to decrease cardiovascular risk. Reducing salt intake may be a simple nonpharmacological way to decrease nocturnal BP, particularly in older individuals who are likely to be salt sensitive. As salt sensitivity causes excessive body fluid retention, it may consequently increase sleep BP by carrying over sodium excretion and natriuresis into the sleep period [14,15,16].
There were several study limitations that warrant mention. First, the frequency of nocturnal urination was determined using a sleep diary with potentially associated misclassifications. However, the use of multiday urination records potentially allowed for the minimization of such misclassifications. Second, antihypertensive drug classes used by participants were not considered. The association between nocturnal urination and baPWV in individuals taking antihypertensive drugs, particularly drugs with vasodilatory action or diuresis, may be slightly different between individuals. Third, study participants were recruited from the general Japanese population, for which salt intake has been reported to be higher than in Western countries [17]. Studies in other populations with different lifestyle habits may strengthen the present findings.
In conclusion, frequent nocturnal urination may represent a potential risk for arterial stiffness, independent of office and sleep BP. Careful attention should be paid to this common phenomenon in older populations with the purpose of not only maintaining quality of life and mental equanimity but also preventing cardiovascular disease.
References
Dani H, Esdaille A, Weiss JP. Nocturia: aetiology and treatment in adults. Nat Rev Urol. 2016;13:573–83. https://doi.org/10.1038/nrurol.2016.134
Breyer BN, Shindel AW, Erickson BA, Blaschko SD, Steers WD, Rosen RC. The association of depression, anxiety and nocturia: a systematic review. J Urol. 2013;190:953–7. https://doi.org/10.1016/j.juro.2013.03.126
Pinggera GM, Mitterberger M, Steiner E, Pallwein L, Frauscher F, Aigner F, et al. Association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: assessment using colour Doppler ultrasonography. BJU Int. 2008;102:470–4. https://doi.org/10.1111/j.1464-410X.2008.07587.x
Bursztyn M, Jacob J, Stessman J. Usefulness of nocturia as a mortality risk factor for coronary heart disease among persons born in 1920 or 1921. Am J Cardiol. 2006;98:1311–5. https://doi.org/10.1016/j.amjcard.2006.06.024
Matsumoto T, Tabara Y, Murase K, Setoh K, Kawaguchi T, Nagashima S, et al. Nocturia and increase in nocturnal blood pressure: the Nagahama study. J Hypertens. 2018;36:2185–92. https://doi.org/10.1097/HJH.0000000000001802
Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension 2011;57:3–10. https://doi.org/10.1161/HYPERTENSIONAHA.109.133900
Vasunta RL, Kesäniemi YA, Ylitalo A, Ukkola O. Nondipping pattern and carotid atherosclerosis in a middle-aged population: OPERA Study. Am J Hypertens. 2012;25:60–6. https://doi.org/10.1038/ajh.2011.159
Tabara Y, Ikezoe T, Yamanaka M, Setoh K, Segawa H, Kawaguchi T, et al. Advanced glycation end product accumulation is associated with low skeletal muscle mass, weak muscle strength, and reduced bone density: The Nagahama Study. J Gerontol A Biol Sci Med Sci. 2018. https://doi.org/10.1093/gerona/gly233.
Tabara Y, Setoh K, Kawaguchi T, Takahashi Y, Kosugi S, Nakayama T, et al. Factors affecting longitudinal changes in cardio-ankle vascular index in a large general population: the Nagahama study. J Hypertens. 2018;36:1147–53. https://doi.org/10.1097/HJH.0000000000001672
Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–64.
Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–7. https://doi.org/10.1097/HJH.0b013e32832e94e7
Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, et al. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19:401–6. https://doi.org/10.1038/sj.jhh.1001838
Imai Y, Kario K, Shimada K, Kawano Y, Hasebe N, Matsuura H, et al. The Japanese Society of Hypertension Guidelines for Self-monitoring of Blood Pressure at Home (Second Edition). Hypertens Res. 2012;35:777–95. https://doi.org/10.1038/hr.2012.56
Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, Nakamura S, et al. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis. 1999;33:29–35.
Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 1997;96:1859–62.
Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 1999;100:1635–8.
Kesteloot H, Tzoulaki I, Brown IJ, Chan Q, Wijeyesekera A, Ueshima H, et al. Relation of urinary calcium and magnesium excretion to blood pressure: The International Study Of Macro- And Micro-nutrients And Blood Pressure and The International Cooperative Study On Salt, Other Factors, And Blood Pressure. Am J Epidemiol. 2011;174:44–51. https://doi.org/10.1093/aje/kwr049
Acknowledgements
We are extremely grateful to the Nagahama City Office and to the nonprofit organization Zeroji Club for their assistance in performing the Nagahama study. We also thank the editors at Enago for the English language review.
Financial Support
The study was supported by a university grant; the Center of Innovation Program; the Global University Project; a Grant-in-Aid for Scientific Research (25293141, 26670313, 26293198, 17H04182, 17H04126, 17H04123, 18K18450) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Practical Research Project for Rare/Intractable Diseases (ek0109070, ek0109070, ek0109196, ek0109348); the Comprehensive Research on Aging and Health Science Research Grants for Dementia R&D (dk0207006, dk0207027); the Program for an Integrated Database of Clinical and Genomic Information (kk0205008); the Practical Research Project for Lifestyle-related Diseases including Cardiovascular Diseases and Diabetes Mellitus (ek0210066, ek0210096, ek0210116); the Research Program for Health Behavior Modification by Utilizing IoT (le0110005) from the Japan Agency for Medical Research and Development (AMED); Welfare Sciences Research Grants; Research on Region Medical from the Ministry of Health, Labor and Welfare of Japan; the Takeda Medical Research Foundation; and the Mitsubishi Foundation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The Department of Respiratory Care and Sleep Control Medicine is funded by endowments from Philips Respironics, Teijin Pharma, ResMed, and Fukuda Lifetec-Keiji through Kyoto University.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabara, Y., Matsumoto, T., Murase, K. et al. Frequent nocturnal urination in older men is associated with arterial stiffness: The Nagahama study. Hypertens Res 42, 1996–2001 (2019). https://doi.org/10.1038/s41440-019-0309-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0309-4
Keywords
This article is cited by
-
Cardiovascular risk independently predicts small functional bladder storage capacity
International Urology and Nephrology (2021)
-
Nocturia is Associated with High Atherosclerotic Cardiovascular Disease Risk in Women: Results from the National Health and Nutrition Examination Survey
Journal of Community Health (2021)