Abstract
Tubulointerstitial damage is a crucial therapeutic target in preventing chronic kidney disease (CKD) progression. Inappropriately activated renin–angiotensin–aldosterone system (RAAS) in the tubulointerstitial area is strongly associated with tubulointerstitial damage progression. Therefore, this study aimed to determine whether there is a beneficial effect of voluntary running exercise training on aldosterone-induced renal injury. Human L-type fatty acid-binding protein (L-FABP) chromosomal transgenic (L-FABP+/−) male mice were used to evaluate the effect of exercise by using urinary L-FABP, a tubular marker. The mice were assigned to either the exercise group that performed voluntary running exercise training using a running wheel or the control group. Subsequently, two groups were injected with aldosterone (0.125 μg kg−1 min−1) and administered 1% NaCl water, and two groups were administered aldosterone only in solvent 4 weeks after initiating the exercise. Aldosterone was injected for another 4 weeks, and NaCl water was administered from 5 weeks after starting the exercise until 8 weeks. Although both aldosterone and NaCl water significantly decreased the running distance, tubulointerstitial damage involving interstitial infiltration of macrophages and fibrosis and the elevation of urinary human L-FABP induced by aldosterone injection was prevented by voluntary running exercise training. Urinary human L-FABP levels were significantly correlated with the degree of tubulointerstitial damage. In conclusion, voluntary running exercise training delayed tubulointerstitial damage progression in the aldosterone-induced renal injury model and therefore may be a promising nonpharmacological strategy in CKD.
Similar content being viewed by others
Introduction
Compared with the degree of glomerular damage, tubulointerstitial damage is widely known to be strongly associated with chronic kidney disease (CKD) progression [1, 2]. Inappropriate activation of the renin–angiotensin–aldosterone system (RAAS) in the tubulointerstitial area is a crucial aggravating factor for CKD progression [3], and RAAS inhibition should be the first-line therapy to prevent aggravation of CKD in clinical practice. However, various side effects induced by RAAS inhibition, such as hypotension, hyperkalemia, and decreased glomerular filtration, make complete RAAS inhibition impossible in patients with CKD. Therefore, another strategy is needed to improve tubulointerstitial damage due to excessive RAAS activation.
Recently, studies have focused on several beneficial effects of exercise training to prevent CKD progression [4, 5]. A prospective study with a large cohort revealed that high-intensity physical activity might decrease the risk of the onset of diabetic nephropathy in patients with type 1 diabetes [6]. Similar to the mechanisms of the effects of exercise training against CKD, some experimental studies showed that exercise induces a protective endothelial function via an anti-inflammatory effect [7, 8] with the potential for improving renal microcirculation due to increased production of a vasodilator, nitric oxide (NO), which is synthesized by activation of endothelial NO synthase (eNOS) [9,10,11] and an anti-oxidative effect [7, 12, 13]. Various factors, such as inflammation, abnormalities of renal hemodynamics, and oxidative stress, are linked to tubulointerstitial damage progression under the excessive activation of RAAS [14,15,16], and thus, exercise training may be a nonpharmacologically useful strategy against tubulointerstitial damage in CKD.
In the current study, the usefulness of voluntary running exercise training on tubulointerstitial damage due to RAAS activation was examined using an aldosterone-induced model of renal injury, which is established by continuous injection of aldosterone (Aldo), the end-product of RAAS, and high salt intake. In the model, human L-type fatty acid-binding protein (L-FABP), which is expressed in human proximal tubules, was revealed to have a renoprotective role against tubulointerstitial damage [17]. Furthermore, we previously reported that levels of urinary human L-FABP, a tubular marker reflecting the degree of renal microcirculation [18, 19], in addition to the degree of oxidative stress and severity of tubulointerstitial damage [20,21,22], were significantly correlated with exercise capacity [23] and decreased by exercise training in humans [24]. Therefore, to investigate whether voluntary running exercise training combined with the expression of human L-FABP prevents the progression of tubulointerstitial damage and whether renal histological findings and urinary L-FABP are changed by exercise training, we used human L-FABP chromosomal transgenic mice because L-FABP is not expressed in mouse kidneys [25].
Materials and methods
Animals
Human L-FABP chromosomal transgenic (L-FABP-Tg) mice on a C57/BL6 background were used to evaluate the degree of tubulointerstitial damage by using urinary L-FABP, a tubular marker, in this study. The human L-FABP-Tg mice were generated by microinjection of the genomic DNA of human L-FABP, including its promoter region, as previously described (patent WO0073791; World Intellectual Property Organization, Switzerland) [26]. The present study was performed in accordance with the St. Marianna University School of Medicine Institutional Guide for Animal Experiments and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, USA).
Experimental design
The current study consisted of an 8-week observation period. At first, the human L-FABP-Tg mice (8-week-old to 10-week-old male mice, n = 34) were divided into four groups as follows: Control (n = 12), Exercise (n = 4), Aldosterone (Aldo) (n = 9), and Exercise–Aldosterone (Ex-Aldo) groups (n = 9). The mice in the Exercise and Ex-Aldo groups performed voluntary running exercise training for 8 weeks. The mice in the Aldo and Ex-Aldo groups were injected with aldosterone 4 weeks after starting the exercise until 8 weeks, and 1% NaCl water was administered 5 weeks after starting the exercise (1 week after starting aldosterone injection) until 8 weeks. During the experimental periods, all mice were housed overnight individually in metabolic cages to collect the urine at weeks 0, 4, and 8 and had free access to tap water. Body weight and blood pressure were evaluated biweekly. The kidneys, skeletal muscles (gastrocnemius), and serum were collected at week 8 for various analyses.
Aldosterone-induced renal injury
Aldosterone-induced renal injury has been established by both systemic infusion of aldosterone (0.125 μg−1 kg−1 min−1, A9477; Sigma-Aldrich, MO, USA) and drinking of 1% NaCl water. The aldosterone was dissolved in polyethylene glycol (PEG 300; Sigma-Aldrich, MO, USA) and infused via an osmotic minipump (Alzet 1004; Durect, CA, USA) implanted into the subcutaneous space of mice anesthetized with isoflurane as previously described [27]. In contrast, both the Control and Exercise groups were infused with vehicle only using the osmotic minipump.
Voluntary running exercise training
Voluntary running exercise training was conducted using a running wheel (ENV-044; Med Associates, VT, USA) for 8 weeks. The number of rotations and running distance were automatically recorded using a Bluetooth device (SOF-860; Med Associates).
Blood pressure
Blood pressure was measured biweekly by using a tail-cuff apparatus (Softron BP-98A; Softron, Tokyo, Japan). Systolic blood pressure values were derived from the mean of three measurements per animal at each time point.
Serum and urinary biochemistry
Serum potassium and sodium levels were measured using an electrode method provided by the clinical laboratory testing services of SRL (Tokyo, Japan). Serum cystatin C levels were evaluated using a sandwich enzyme-linked immunosorbent assay (ELISA) method (Cystatin C Mouse ELISA kit; BioVendor Laboratory Medicine, Modrice, Czech Republic). Serum and urinary creatinine levels were measured using the Jaffe method (Creatinine Assay Kit; BioAssay Systems, CA, USA). Urinary albumin levels were determined using a Mouse Albumin ELISA kit (Albuwell M; Exocell, Inc., PA, USA). Urinary human L-FABP levels were measured using a two-step sandwich ELISA procedure (human L-FABP ELISA kit, CMIC, Tokyo, Japan) as previously described [21]. All urinary parameters are reported as ratios relative to urinary creatinine levels.
Renal histologic and morphometric analysis
For renal histologic and morphometric analysis, the kidneys were fixed in methyl Carnoy’s solution or 10% buffered formalin, dehydrated, and embedded in paraffin. Some sections (3-μm thick) were obtained for renal histological assessments, such as periodic acid–Schiff staining and immunohistochemistry. Tubulointerstitial damage in periodic acid–Schiff-stained sections was categorized as tubular dilation with epithelial and tubular atrophy. Under magnification (×100), 7–10 nonoverlapping fields from the entire cortical and outer medulla areas were selected. Tubulointerstitial damage areas were measured manually using an image analyzer (version 6.4, Auto/Manual Measurement Software, WinRoof, Mitani, Tokyo, Japan). The degree of tubulointerstitial damage was evaluated as ratios relative to the entire cortical and outer medulla areas [27].
Immunohistologic analysis
Using the sections preprocessed as described above, an indirect immunoperoxidase method was used to identify the antigens, as previously described [20]. Macrophage infiltration was identified using rat monoclonal antibody F4/80 (1:200, BMA Biomedicals, Augst, Switzerland). Collagen types I and III were identified using rabbit polyclonal antibodies (1:200, Southern Biotech, AL, USA). For immunohistological assessment of myofibroblasts, tissue specimens fixed in 10% buffered formalin and embedded in paraffin were immunostained using a mouse monoclonal antibody to α-smooth muscle actin (α-SMA) (1:800, Sigma-Aldrich, MO, USA).
Visualization was performed by incubation with polymeric horseradish peroxidase-conjugated secondary antibodies (ready-to-use, ImmPRESS Polymer Detection kit, Vector Laboratories, CA, USA). Peroxidase activity was revealed by a diaminobenzidine reaction (Liquid DAB+, DAKO Japan, Tokyo, Japan), and sections were counterstained with hematoxylin. We selected 7–10 nonoverlapping fields from the entire cortical and outer medulla areas. The degree of macrophage infiltration and interstitial myofibroblasts in the cortical and outer medulla interstitium were automatically measured by separating positively stained areas of F4/80 and α-SMA and are expressed as ratios of F4/80-positive and α-SMA-positive areas relative to the entire cortical and outer medulla areas under 100× magnification, as measured with the image analyzer (WinRoof). Similarly, the positive areas for collagen types I and III were automatically measured by separating positively stained areas for collagen types I and III and are expressed as ratios of collagen type I-positive and III-positive areas relative to the entire cortical and outer medulla areas.
Statistical analysis
All data are shown as the means ± SE. The differences between the four groups at week 8 were analyzed using one-way analysis of variance with the Tukey–Kramer test, as the sample numbers were different among the groups. The relationships between urinary human L-FABP levels and the degree of tubulointerstitial damage and systolic blood pressure were analyzed using Spearman’s rank correlation coefficients (rs). Statistical significance was set a priori at P < 0.05 for all comparisons. Statistical analyses were performed using SPSS software (version 21).
Results
Running distance
Figure 1a shows time-related changes in the mean running distance in the Exercise and Ex-Aldo groups. The mean running distance of both groups gradually increased from week 1 to week 4. The mean running distance of both groups gradually decreased after the osmotic minipump was injected (at week 4). Furthermore, the average running distance in the Ex-Aldo group notably decreased after drinking 1% NaCl water, and significant differences were found between the groups in weeks 6, 7, and 8.
Time-related changes in the mean running distance (a) and systolic blood pressure (b). Control, n = 12; Exercise, n = 4; Aldosterone (Aldo), n = 9; Exercise–Aldosterone (Ex-Aldo), n = 9. Values are represented as the mean ± SE. ‡P < 0.001 vs. control group, ¶P < 0.05 vs. Ex-Aldo group, §P < 0.05 vs. exercise group
Systolic blood pressure
Time-related changes in systolic blood pressure are presented in Fig. 1b. From week 0 to week 4, the systolic blood pressure levels were similar in all four groups. The systolic blood pressure levels increased in the Aldo and Ex-Aldo groups after systemic aldosterone infusion and drinking 1% NaCl water. The systolic blood pressure levels were significantly higher in the Aldo group than in the other three groups at week 8.
Animal characteristic findings
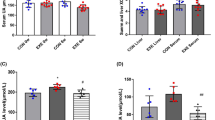
Table 1 outlines the animal characteristic findings, including body weight, renal and muscle weight, serum biochemistry findings, urine volume, and total and mean running distance at week 8. The body weight in the exercise and Ex-Aldo groups was significantly lower than that in the control group (both P < 0.05), and the body weight in the Ex-Aldo group was significantly lower than that in the Aldo group (P < 0.05). The renal weights in the Aldo and Ex-Aldo groups were significantly higher than those in the control group (P < 0.001). In contrast, the muscle weight was similar in all four groups.
The serum creatinine levels in the Aldo group were significantly higher than those in the control group (P < 0.05). Although the serum creatinine levels were significantly lower in the Ex-Aldo group than in the Aldo group (P < 0.05), serum cystatin C levels in the Ex-Aldo group were similar to those in the Aldo group and were significantly lower than those in the control group (P < 0.05). The serum sodium levels in the Aldo and Ex-Aldo mice were significantly higher than those in the control mice (both P < 0.001). The serum potassium levels in the Aldo group were significantly lower than those in the control group (P < 0.05).
The urine volume tended to be higher in the Aldo group and was significantly higher in the Ex-Aldo group compared with the control group (P < 0.05). The total and mean running distance tended to be lower in the Ex-Aldo group compared with the exercise group.
Urinary biochemistry findings
Figure 2 shows the urinary biochemistry findings at week 8. Urinary human L-FABP levels in the Aldo and Ex-Aldo mice at week 8 were significantly higher than those in the control mice (P < 0.001, P < 0.05, respectively; Fig. 2a). Furthermore, the urinary human L-FABP levels in the Ex-Aldo group were significantly lower than those in the Aldo group (P < 0.05; Fig. 2a). The urinary albumin levels in the Aldo and Ex-Aldo mice at week 8 were also significantly higher than those in the control mice (both P < 0.001; Fig. 2b), and the levels in the Ex-Aldo group were significantly lower than those in the Aldo group (P < 0.05; Fig. 2b). In contrast, both urinary sodium levels (Fig. 2c) and urinary potassium levels (Fig. 2d) in the Aldo and Ex-Aldo mice at week 8 were significantly higher than those in the control mice (both P < 0.05).
Urinary biochemistry findings at week 8 (a, b, c, d). Control, n = 12; Exercise, n = 4; Aldosterone (Aldo), n = 9; exercise-aldosterone (Ex-Aldo), n = 9. aData available as follows: Control, n = 9; Exercise, n = 4; Aldo, n = 7; Ex-Aldo, n = 9. P-values were evaluated using one-way ANOVA with a post hoc Tukey–Kramer test. Values are presented as the mean ± SE. **P < 0.001, *P < 0.05. L-FABP, L-type fatty acid-binding protein
Renal histologic and morphometric analysis
Periodic acid–Schiff-stained sections revealed tubulointerstitial damage, including dilatation of tubules and degeneration of proximal tubular epithelial cells in both the Aldo and Ex-Aldo groups. However, the degree of tubulointerstitial damage in the Ex-Aldo mice was significantly lower than that in the Aldo mice (P < 0.05; Fig. 3a, b).
Histological staining with periodic acid–Schiff showing tubulointerstitial damage (a, b). Control, n = 12; exercise, n = 4; Aldosterone (Aldo), n = 9; exercise-aldosterone (Ex-Aldo), n = 9. P-values were evaluated using one-way ANOVA with a post hoc Tukey–Kramer test. Values are presented as the mean ± SE. **P < 0.001, *P < 0.05
Evaluation of macrophage infiltration
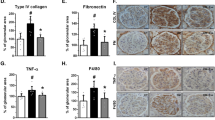
The degree of tubulointerstitial inflammation was evaluated by macrophage infiltration via immunohistochemical analysis using the rat monoclonal antibody F4/80 (Fig. 4a, b). Macrophage infiltration into the kidney in both Aldo and Ex-Aldo mice was significantly higher than that in the control mice (both P < 0.001); however, the degree of tubulointerstitial inflammation in the Ex-Aldo mice was significantly lower than that in the Aldo mice (P < 0.05).
Immunohistological staining using an antibody against F4/80 (a, b), α-smooth muscle actin (α-SMA) (c, d), type I collagen (e, f), and type III collagen (g, h). Control, n = 12; exercise, n = 4; aldosterone (Aldo), n = 9; exercise-aldosterone (Ex-Aldo), n = 9. P-values were evaluated using one-way ANOVA with a post hoc Tukey–Kramer test. Values are presented as the mean ± SE. **P < 0.001, *P < 0.05
Evaluation of tubulointerstitial fibrosis
The degree of tubulointerstitial fibrosis was assessed by α-SMA and types I and III collagen expression using immunohistochemical analysis. The α-SMA-positive cells (Fig. 4c, d) and deposition levels of types I (Fig. 4e, f) and III (Fig. 4g, h) collagen in the kidneys in both Aldo and Ex-Aldo mice were significantly higher than those in the control mice (P < 0.001). However, the degree of tubulointerstitial fibrosis (α-SMA-positive cells and deposition levels of types I and III collagen) in the Ex-Aldo mice was significantly lower than that in the Aldo mice (P < 0.001, P < 0.05, and P < 0.05, respectively).
Relationship between urinary human L-FABP levels and the degree of tubulointerstitial damage, inflammation, and fibrosis
Urinary human L-FABP levels at week 8 were significantly correlated with tubulointerstitial damage in periodic acid–Schiff-stained sections (rs = 0.785, P < 0.001; Fig. 5a), the degree of tubulointerstitial inflammation by immunohistological staining using an antibody against F4/80 (rs = 0.783, P < 0.001; Fig. 5b), and the degree of tubulointerstitial fibrosis evaluated by α-SMA (rs = 0.818, P < 0.001; Fig. 5c), type I (rs = 0.759, P < 0.001; Fig. 5d) and III collagen (rs = 0.723, P < 0.001; Fig. 5e) expression using immunohistochemical analysis.
Relationship between urinary human L-type fatty acid-binding protein (L-FABP) levels and tubulointerstitial damage by periodic acid–Schiff staining (a), F4/80-positive area (b), α-smooth muscle actin (αSMA)-positive area (c), type I collagen-positive area (d), type III collagen-positive area (e). aData available for 29 animals
Discussion
In the current study, we showed the effects of voluntary running exercise training as well as the expression of human L-FABP on histological tubulointerstitial damage in an aldosterone-induced renal injury model using human L-FABP-Tg mice. Furthermore, the elevation of urinary L-FABP levels was suppressed by exercise in the model. These results suggest the possibility of exercise as another strategy to improve tubulointerstitial damage due to excessive activation of RAAS in clinical practice.
Both angiotensin II and aldosterone produced by RAAS activation play a central role in the progression of tubulointerstitial damage [28, 29]. Agarwal et al. reported that forced exercise training using a motor-driven treadmill for 16 weeks attenuated both glomerular and tubulointerstitial damage in spontaneously hypertensive rats [7], in which renal damage was attenuated by angiotensin II receptor blockers without a reduction in blood pressure [30]. Furthermore, the renoprotective effects of the combination of exercise training and antihypertensive therapy have been reported in several other CKD rat models [31, 32]. In addition to these studies, our study showed that voluntary running exercise training prevented tubulointerstitial damage progression in an aldosterone-induced renal injury mouse model, in which renal damage was attenuated by the mineralocorticoid receptor blocker [17]. Taken together, the previous and current results suggest the importance of exercise as a nonpharmacological strategy in CKD for several reasons, including RAAS activation.
The mechanisms underlying the renoprotective effects of exercise training are still not fully clarified, although several factors might be involved. Several previous studies have demonstrated that anti-inflammatory [7, 8] and anti-oxidative action [7, 12, 13], which is known to prevent CKD progression due to RAAS activation, participated in the beneficial effects of exercise training. We analyzed the differences in gene expression between the kidneys of the mice with voluntary running exercise training for 8 weeks and those of the mice without the exercise by microarray (Cell Innovator, Fukuoka, Kyushu, Japan) and found a significant decrease in the expression of the plasminogen activator inhibitor (PAI-1) gene, one of the well-known mediators in the pathogenesis of renal fibrosis, in the kidneys of the mice with exercise compared with those without exercise (data not shown). PAI-1 expression was reported to decrease with the improvement in tubulointerstitial damage in the aldosterone-induced renal injury model [33]. Therefore, we examined renal PAI-1 gene expression using real-time PCR analysis in our models. The PAI-1 gene expression that was upregulated in both Aldo and Ex-Aldo mice tended to be lower in the Ex-Aldo mice than in the Aldo mice (P = 0.128; Supplementary Fig. 1A). Furthermore, the PAI-1 gene expression levels at week 8 tended to be negatively correlated with running distance (rs = −0.396, P = 0.180). There is a possibility that exercise may prevent the increase in renal PAI-1 expression, leading to amelioration of tubulointerstitial damage in the Aldo model. Regarding the reduction in oxidative stress by exercise, because oxidative stress is produced by NADPH oxidase, we examined the gene expression of p47-phox (NCF1), a cytosolic subunit of the multiprotein complex forming NADPH oxidase, and obtained the same results as those of PAI-1 expression (P = 0.436; Supplementary Fig. 1B). A decrease in oxidative stress by voluntary running exercise training might lead to attenuation of tubulointerstitial damage. Alternatively, the eNOS-NO pathway accelerates local inflammation by increasing vascular permeability to allow access to inflammatory cells, such as macrophages. Furthermore, Aldo induces the upregulation of eNOS expression in endothelial cells [34, 35]. Our results showed that eNOS gene expression in both the Aldo and Ex-Aldo mice was significantly higher than that in the control mice (P < 0.001) and that the eNOS expression in the Ex-Aldo mice tended to be lower than that in the Aldo mice (P = 0.490; data not shown). These results showed that the eNOS-NO pathway might not contribute to the renoprotective effect caused by exercise in the Aldo model.
Momentum (i.e., running distance) gradually decreased after injection with aldosterone and NaCl water in the Ex-Aldo group. This result coincides with the results of the human observational study indicating that physical activity decreases with kidney disease progression [36]. Hence, securing sufficient momentum in both humans and mice will be difficult based on the degree of kidney disease. Even with less momentum, however, tubulointerstitial damage was significantly reduced in the Ex-Aldo group compared with the Aldo group. Therefore, continuing exercise might be important when considering a preventive strategy for CKD progression.
The current study has several critical limitations. First, the human L-FABP-Tg mice used in this study have been reported to have enhanced anti-oxidative effects and reduced tubulointerstitial damage progression compared with the wild-type mice without L-FABP expression in their proximal tubules [17]. Thus, several of the beneficial effects demonstrated in this study might have been affected by human L-FABP expression as well as voluntary running exercise training. Accordingly, an investigation using wild-type mice is required to reveal the renoprotective effects of exercise training on the aldosterone-induced renal injury model. However, because L-FABP is expressed in human kidneys, it is important to study the renoprotective role of exercise in addition to the L-FABP expression in the present renal injury model before trying a clinical application of exercise therapy in CKD patients. Second, although we examined the possible factors (e.g., monocyte chemotactic protein-1, etc.) related to the mechanism underlying the beneficial effects of exercise training on the kidneys, the crucial mechanistic factors were not completely clarified in this study. However, we think that the results of several previous studies and our present study suggest the possibility that a number of pathways and small changes in many factors, rather than one specific pathway, were involved in the mechanism underlying the renoprotective effect caused by exercise. Furthermore, several beneficial effects of exercise training on the kidneys might be associated with not only the intrarenal but also the extrarenal factors (e.g., myokines). Indeed, a recent previous study reported the possibility that some myokines were involved in renal injury prevention [37]. Finally, we cannot suggest the most effective exercise training (intensity, duration, and frequency) for kidney health because the current study investigated only the effects of “8 weeks” of “voluntary” exercise training. Therefore, further studies are needed to investigate the more detailed underlying mechanisms and the optimization of exercise training to recommend optimal exercise to patients with CKD. Alternatively, the present study showed that urinary L-FABP levels decreased with the improvement in tubulointerstitial damage induced by exercise and that the levels were significantly correlated with the degree of tubulointerstitial damage, interstitial inflammation, and renal fibrosis, which supported the results reported in previous studies. Furthermore, urinary human L-FABP is a biomarker promulgated by the Ministry of Health, Labor, and Welfare in Japan. Therefore, urinary L-FABP may be useful not only for the evaluation of the renoprotective effect of exercise but also for deciding suitable effective exercise training in clinical practice.
In conclusion, the current study showed that voluntary running exercise training suppressed tubulointerstitial damage progression and elevation of urinary human L-FABP in an aldosterone-induced renal injury model. Although further study is needed to reveal the optimal exercise training program to prevent CKD, performing exercise habitually might be beneficial for kidney health and may be a promising nonpharmacological strategy for CKD patients.
References
Prunotto M, Budd DC, Gabbiani G, Meier M, Formentini I, Hartmann G, et al. Epithelial-mesenchymal crosstalk alteration in kidney fibrosis. J Pathol. 2012;228:131–47.
Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–56.
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28.
Hama T, Oikawa K, Ushijima A, Morita N, Matsukage T, Ikari YJ, et al. Effect of cardiac rehabilitation on the renal function in chronic kidney disease—analysis using serum cystatin-C based glomerular filtration rate. Int J Cardiol Heart Vasc. 2018;19:27–33.
Greenwood SA, Koufaki P, Mercer TH, MacLaughlin HL, Rush R, Lindup H, et al. Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis. 2015;65:425–34.
Waden J, Tikkanen HK, Forsblom C, Harjutsalo V, Thorn LM, Saraheimo M, et al. Leisure-time physical activity and development and progression of diabetic nephropathy in type 1 diabetes: the FinnDiane Study. Diabetologia. 2015;58:929–36.
Agarwal D, Elks CM, Reed SD, Mariappan N, Majid DS, Francis J. Chronic exercise preserves renal structure and hemodynamics in spontaneously hypertensive rats. Antioxid Redox Signal. 2012;16:139–52.
Fukao K, Shimada K, Naito H, Sumiyoshi K, Inoue N, Iesaki T, et al. Voluntary exercise ameliorates the progression of atherosclerotic lesion formation via anti-inflammatory effects in apolipoprotein E-deficient mice. J Atheroscler Thromb. 2010;17:1226–36.
Ito D, Cao P, Kakihana T, Sato E, Suda C, Muroya Y, et al. Chronic running exercise alleviates early progression of nephropathy with Upregulation of nitric oxide synthases and suppression of glycation in Zucker diabetic rats. PLoS ONE. 2015;10:e0138037.
Ito D, Ito O, Mori N, Cao P, Suda C, Muroya Y, et al. Exercise training upregulates nitric oxide synthases in the kidney of rats with chronic heart failure. Clin Exp Pharm Physiol. 2013;40:617–25.
Ito D, Ito O, Cao P, Mori N, Suda C, Muroya Y, et al. Effects of exercise training on nitric oxide synthase in the kidney of spontaneously hypertensive rats. Clin Exp Pharm Physiol. 2013;40:74–82.
George L, Lokhandwala MF, Asghar M. Exercise activates redox-sensitive transcription factors and restores renal D1 receptor function in old rats. Am J Physiol Ren Physiol. 2009;297:F1174–1180.
Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Ren Physiol. 2007;293:F914–919.
Irita J, Okura T, Jotoku M, Nagao T, Enomoto D, Kurata M, et al. Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am J Physiol Ren Physiol. 2011;301:F833–844.
Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–8.
Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800.
Ichikawa D, Kamijo-Ikemori A, Sugaya T, Shibagaki Y, Yasuda T, Hoshino S, et al. Human liver-type fatty acid-binding protein protects against tubulointerstitial injury in aldosterone-induced renal injury. Am J Physiol Ren Physiol. 2015;308:F114–121.
Imai N, Yasuda T, Kamijo-Ikemori A, Shibagaki Y, Kimura K. Distinct roles of urinary liver-type fatty acid-binding protein in non-diabetic patients with anemia. PLoS ONE. 2015;10:e0126990.
Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, et al. Renal L-type fatty acid–binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–902.
Ichikawa D, Kamijo-Ikemori A, Sugaya T, Ohata K, Hisamichi M, Hoshino S, et al. Utility of urinary tubular markers for monitoring chronic tubulointerstitial injury after ischemia-reperfusion. Nephrology. 2018;23:308–16.
Ohata K, Kamijo-Ikemori A, Sugaya T, Hibi C, Nakamura T, Murase T, et al. Renoprotective effect of the xanthine oxidoreductase inhibitor Topiroxostat under decreased angiotensin II type 1a receptor expression. Eur J Pharm. 2017;815:88–97.
Yokoyama T, Kamijo-Ikemori A, Sugaya T, Hoshino S, Yasuda T, Kimura K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol. 2009;174:2096–106.
Kosaki K, Kamijo-Ikemori A, Sugaya T, Tanahashi K, Kumagai H, Sawano Y, et al. Relationship between exercise capacity and urinary liver-type fatty acid-binding protein in middle-aged and older individuals. Clin Exp Nephrol. 2017;21:810–7.
Kosaki K, Kamijo-Ikemori A, Sugaya T, Tanahashi K, Sawano Y, Akazawa N, et al. Effect of habitual exercise on urinary liver-type fatty acid-binding protein levels in middle-aged and older adults. Scand J Med Sci Sports. 2018;28:152–60.
Simon TC, Roth KA, Gordon JI. Use of transgenic mice to map cis-acting elements in the liver fatty acid-binding protein gene (Fabpl) that regulate its cell lineage-specific, differentiation-dependent, and spatial patterns of expression in the gut epithelium and in the liver acinus. J Biol Chem. 1993;268:18345–58.
Kamijo A, Sugaya T, Hikawa A, Okada M, Okumura F, Yamanouchi M, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165:1243–55.
Hisamichi M, Kamijo-Ikemori A, Sugaya T, Hoshino S, Kimura K, Shibagaki Y. Role of bardoxolone methyl, a nuclear factor erythroid 2-related factor 2 activator, in aldosterone- and salt-induced renal injury. Hypertens Res. 2018;41:8–17.
Kadoya H, Satoh M, Sasaki T, Taniguchi S, Takahashi M, Kashihara N. Excess aldosterone is a critical danger signal for inflammasome activation in the development of renal fibrosis in mice. FASEB J. 2015;29:3899–910.
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharm Rev. 2007;59:251–87.
Susic D, Frohlich ED, Kobori H, Shao W, Seth D, Navar LG. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J Hypertens. 2011;29:716–23.
Tufescu A, Kanazawa M, Ishida A, Lu H, Sasaki Y, Ootaka T, et al. Combination of exercise and losartan enhances renoprotective and peripheral effects in spontaneously type 2 diabetes mellitus rats with nephropathy. J Hypertens. 2008;26:312–21.
Kanazawa M, Kawamura T, Li L, Sasaki Y, Matsumoto K, Kataoka H, et al. Combination of exercise and enalapril enhances renoprotective and peripheral effects in rats with renal ablation. Am J Hypertens. 2006;19:80–86.
Wang B, Ding W, Zhang M, Li H, Gu Y. Rapamycin attenuates aldosterone-induced tubulointerstitial inflammation and fibrosis. Cell Physiol Biochem. 2015;35:116–25.
Lyngso KS, Assersen K, Dalgaard EG, Skott O, Jensen BL, Hansen PB. Does aldosterone play a significant role for regulation of vascular tone? J Cardiovasc Pharm. 2016;68:1–10.
Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, et al. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48:165–71.
Zelle DM, Klaassen G, van Adrichem E, Bakker SJ, Corpeleijn E, Navis G. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol. 2017;13:152–68.
Hanatani S, Izumiya Y, Araki S, Rokutanda T, Kimura Y, Walsh K, et al. Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J Am Soc Nephrol. 2014;25:2800–11.
Acknowledgements
We thank Ms. Kimie Katayama and Ms. Junko Asano for technical assistance.
Funding
This study was supported by a grant-in-Aid for the Japan Society for the Promotion of Science Fellows KAKENHI Grant Number 17J01405, Mitsui Sumitomo Insurance Welfare Foundation, and Akaeda Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TS is the director and a senior scientist, and KO is a scientist at CMIC HOLDINGS Co., Ltd., which produced the kits for L-FABP analysis. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kosaki, K., Sugaya, T., Ohata, K. et al. Renoprotective effects of voluntary running exercise training on aldosterone-induced renal injury in human L-FABP chromosomal transgenic mice. Hypertens Res 42, 1518–1527 (2019). https://doi.org/10.1038/s41440-019-0273-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0273-z
Keywords
This article is cited by
-
Elevated urinary angiotensinogen excretion links central and renal hemodynamic alterations
Scientific Reports (2023)
-
The effects of exercise on kidney injury: the role of SIRT1
Molecular Biology Reports (2022)