Abstract
Accumulating evidence has indicated the potential contributions of aldosterone and mineralocorticoid receptor (MR) to the pathophysiology of cardiovascular disease (CVD) and chronic kidney disease (CKD). Patients with primary aldosteronism have a higher risk of CVD and CKD than those with essential hypertension. MR is strongly expressed in endothelial cells, vascular smooth muscle cells, cardiomyocytes, fibroblasts, macrophages, glomerular mesangial cells, podocytes, and proximal tubular cells. In these cardiovascular and renal cells, aldosterone-induced cell injury is prevented by MR blockade. Interestingly, MR antagonists elicit beneficial effects on CVD and CKD in subjects with low or normal plasma aldosterone levels. Recent studies have shown that during development of CVD and CKD, cardiovascular and renal MR is activated by glucocorticoid and ligand-independent mechanisms, such as Rac1 signaling pathways. These data indicate that inappropriate activation of local MR contributes to cardiovascular and renal tissue injury through aldosterone-dependent and -independent mechanisms. In this review, recent findings on the specific role of cardiovascular and renal MR in the pathogenesis of CVD and CKD are summarized.
Similar content being viewed by others
Introduction
Aldosterone regulates body fluid by activation of mineralocorticoid receptor (MR) in distal tubules and collecting ducts [1, 2]. In addition to the effects of aldosterone on body fluid homeostasis, accumulating evidence suggests that aldosterone and MR contribute to the pathogenesis of cardiovascular disease (CVD) and chronic kidney disease (CKD). Patients with primary aldosteronism (PA) have a higher incidence of cardiovascular complications and albuminuria than do patients with essential hypertension [3,4,5]. The Randomized Aldactone Evaluation Study showed that adding spironolactone, a non-selective MR antagonist, to standard therapy significantly reduced morbidity and mortality in patients with moderate to severe heart failure [6]. Similarly, adding the selective MR antagonist eplerenone reduced morbidity and mortality in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure [7]. Cardiovascular protective effects of MR antagonists have also been reported in patients with chronic dialysis [8]. This finding suggests that these effects of MR antagonists are not mediated by their effects on tubular function. Based on this evidence, many national guideline groups have recommended MR antagonists in preference to other antihypertensive agents in hypertensive patients with CVD [9,10,11]. Addition of MR antagonists to inhibitors of the renin–angiotensin system (RAS), such as angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, reduces albuminuria in patients with non-diabetic CKD [12] or type 2 diabetic kidney disease [13]. However, the frequency and severity of hyperkalemia are significantly increased by administration of currently available steroidal MR antagonists (i.e., spironolactone and eplerenone) [14]. Therefore, spironolactone and eplerenone are widely recommended to be carefully prescribed with closer monitoring, especially in patients with CKD [15]. However, clinical trials are currently being conducted to examine renoprotective effects of several non-steroidal MR blockers in patients with type 2 diabetic kidney disease [16].
To achieve a reduction in risk of CVD and CKD, blood pressure control is essential [17, 18]. In this regard, MR antagonists are frequently effective in patients with PA and in those without PA with resistant hypertension [19,20,21]. Additionally, a potential contribution of MR to insulin resistance [22, 23] and sympathetic nerve activation [24] has been indicated. These MR-induced systemic changes play a critical role in the pathogenesis of CVD and CKD. However, this review focuses on the specific role of locally expressed MR in cardiovascular and renal tissues with particular emphasis on their possible contributions to the pathophysiology of CVD and CKD. Detailed intracellular molecular mechanisms of MR activation have been reviewed by other authors [20, 21, 25, 26] and are not discussed here.
Possible mechanism of MR activation in CVD and CKD
MR activation by inappropriately increased aldosterone levels
Clinical studies have shown that plasma aldosterone levels are significantly associated with the risks of CVD and CKD, even when they do not meet the diagnostic criteria of PA [20]. Interestingly, plasma aldosterone levels are also correlated with body mass index [27], suggesting a possible relationship between aldosterone levels and obesity. Recent studies have shown that several adipocyte-derived aldosterone release factors stimulate aldosterone secretion from the adrenal gland [28, 29]. Therefore, patients with obesity and CVD and CKD might show relatively high aldosterone levels, which may further increase the risks of CVD and CKD via MR activation.
High-risk patients with CVD and CKD are often treated with an RAS inhibitor. Because angiotensin II induces aldosterone release from the adrenal gland, treatment with an RAS inhibitor should reduce plasma aldosterone levels [1]. However, after long-term treatment with RAS inhibitors, originally reduced plasma aldosterone levels return to pretreatment levels again in certain patients [30]. This phenomenon is called aldosterone breakthrough, which attenuates myocardial- and renal-protective effects of RAS inhibitors. In patients with CVD and CKD who show aldosterone breakthrough, addition of an MR antagonist significantly restores the organ-protective effects of RAS inhibitors without changing blood pressure [31, 32]. These data suggest that inappropriately elevated plasma aldosterone levels play a critical role in the pathogenesis of CVD and CKD in high-risk patients who are treated with an RAS inhibitor in the long term.
Aldosterone-independent MR activation
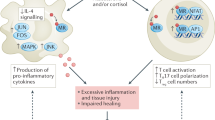
Beneficial effects of MR antagonists on CVD and CVD are found in subjects with low or normal plasma aldosterone levels [20, 33, 34]. Therefore, in some pathophysiological conditions, MR might be activated by an aldosterone-independent mechanism. These mechanisms could involve increases in MR gene transcription, MR sensitivity, MR stabilization, and/or MR stimulation by other factors, including glucocorticoid and MR active mutation [20] (Fig. 1).
Possible mechanism of mineralocorticoid receptor (MR) activation in cardiovascular disease (CVD) and chronic kidney disease (CKD). MR is activated by an aldosterone-independent mechanism. Recently, the role of Rac1 in ligand-dependent and -independent MR activation has been highlighted. RAS renin–angiotensin system, ACTH adrenocorticotropic hormone
The affinity of MR is similar between aldosterone and glucocorticoids, but plasma glucocorticoid levels are 1000 times greater than those of aldosterone [2, 20]. Although 11-hydroxysteroid dehydrogenase type 2 transforms glucocorticoids into inactive metabolites [20], several studies have indicated that physiological levels of glucocorticoids activate MR under pathophysiological conditions [35]. Furthermore, glucocorticoids are increased in subjects with obesity, diabetes, and inflammation [36]. This evidence suggests that inappropriate activation of MR might be induced by glucocorticoids during development of stressful lifestyle-related diseases, such as contemporary CVD and CKD (Fig. 1). Consistent with this hypothesis, we previously demonstrated that glucocorticoid-induced MR activation mediates renal injury in high salt-treated adrenalectomized rats [37].
A recent study showed that Rac1 activates MR in a ligand-dependent and -independent manner [21, 38] (Fig. 1). Rac1 is activated by several factors, including cytokines [39], mechanical stress [40], dietary high-salt intake [41] and oxidative stress [42], all of which are risk factors of CVD and CKD. Therefore, during the development of CVD and CKD, MR is activated by Rac1-dependent pathways, which further increases the risk of CVD and CKD.
Specific role of MR in the pathogenesis of CVD
MR is expressed in several cardiovascular cell types, such as cardiomyocytes, endothelial cells, fibroblasts, and vascular smooth muscle cells (VSMCs). Selective deletion or overexpression of MR in different cells has been performed to clarify the specific roles of MR in each cell, as described below.
Specific role of MR in cardiomyocytes and fibroblasts
To determine the specific role of MR in cardiomyocytes, a transgenic mouse model with conditional cardiomyocyte-specific overexpression of human MR was generated. Interestingly, overexpression of human MR in cardiomyocyte induced severe arrhythmias and a high rate of death by ion channel remodeling, whereas myocardial fibrosis and inflammation were not observed [43]. Furthermore, cardiomyocyte-specific overexpression of human MR impairs the nitric oxide-dependent relaxing response in the coronary artery by an increase in myocardial oxidative stress [44]. These findings suggest the specific contribution of cardiomyocyte MR to arrhythmia and coronary dysfunction. This raises the possibility that effects of MR antagonists on sudden death are mediated by their protective effect on arrhythmia and coronary dysfunction (Fig. 2).
Further studies using cardiomyocyte-specific MR knockout (KO) mice showed that specific deletion of cardiomyocyte MR improved left ventricular (LV) dysfunction after transverse aortic constriction [45]. Similarly, cardiac remodeling after myocardial infarction was attenuated in cardiomyocyte-specific MR-KO mice [46]. Interestingly, these mice showed no changes in cardiac hypertrophy. These data are inconsistent with previous in vitro studies, which showed that aldosterone-induced myocyte hypertrophy was blocked by spironolactone [47]. Moreover, inducible knock-down of MR attenuated cardiac hypertrophy [48]. Further studies are required to clarify the role of MR in myocardial hypertrophy during progression of CVD (Fig. 2).
Specific deletion of MR in cardiomyocytes does not alter cardiac fibrosis after transverse aortic constriction [45]. However, cardiomyocyte-specific MR-KO mice showed less inflammatory cell infiltration and fibrosis when induced by deoxycorticosterone (DOCA) and salt [49]. Specific deletion of MR in macrophages improves vascular remodeling [50] and myocardial fibrosis [51, 52]. Importantly, MR deletion in macrophages reduces cardiac inflammation and fibrosis without changing infiltrating macrophage cell numbers in the heart [51, 52]. These data indicate that MR in macrophages plays a central role in cardiac fibrosis. Detailed mechanisms responsible for activation of MR in macrophages are well summarized in other review articles [53, 54] and not discussed here. Notably, specific deletion of MR in fibroblasts did not change cardiac fibrosis after transverse aortic constriction [45], suggesting no contribution of MR in myocardial fibroblasts to cardiac fibrosis (Fig. 2).
Nagase et al. [42] have shown that oxidative stress activates MR in cardiomyocytes by the ligand-independent Rac1-dependent pathway. Recently, Ayuzawa et al. [55] showed that heterozygous deletion of Rac1 in cardiomyocytes reduces myocardial MR signaling. This was associated with attenuation of transverse aortic constriction-induced oxidative stress and cardiac dysfunction. Because treatment with an MR antagonist also improved transverse aortic constriction-induced cardiac dysfunction, the authors concluded that oxidative stress-stimulated Rac1 plays a role in myocardial dysfunction through MR activation. However, these studies also showed that cardiac hypertrophy was attenuated by heterozygous deletion of Rac1 in cardiomyocytes. As described above, transverse aortic constriction-induced cardiac hypertrophy was not changed by specific deletion of MR in cardiomyocytes [45, 46]. This finding suggests that effects of cardiomyocyte-specific Rac1 deletion on cardiac hypertrophy may be mediated by an MR-independent mechanism.
Specific role of MR in endothelial cells and VSMCs
The finding that MR is expressed in the vasculature (i.e., endothelial cells and VSMCs) raises the question of its role in vascular function and injury. Nguyen Dinh Cat et al. [56] generated a transgenic mouse model with conditional endothelial cell-specific overexpression of human MR. They found increased contractile response of resistance arteries in the absence of vascular morphological changes. These mice also showed mild hypertension and acute blood pressure elevation in response to angiotensin II and endothelin-1 infusion. These findings suggest that MR activation in endothelium increases blood pressure independent of tubular effects of MR.
Specific deletion of MR in endothelial cells attenuates Western diet (high fat and high sucrose)-induced endothelial dysfunction, as well as vascular remodeling [57]. Similarly, coronary arterioles of endothelial cell-specific MR-KO mice show decreased constriction to endothelin-1 and thromboxane [58]. Therefore, improvement of coronary function by MR blockade might contribute to the beneficial effect on myocardial function during development of CVD.
However, a direct role of MR in endothelial cells in myocardial function and remodeling is still controversial. Lother et al. [59] reported that specific deletion of endothelial MR significantly improved DOCA/salt-induced cardiac hypertrophy and fibrotic changes without a change in blood pressure. This was associated with preventing upregulation of vascular cell adhesion molecular 1 gene expression. In contrast, Salvador et al. [60] showed that specific deletion of MR in endothelial cells preserved systolic function, but did not improve cardiac hypertrophy and inflammation induced by pressure overload with transverse aortic constriction. Jia et al. [61] also showed that cardiac stiffness and diastolic function induced by a Western diet was prevented by specific deletion of MR in endothelial cells. Importantly, those three studies used mice that had a Cre recombinase transgene driven by the endothelial cell-specific cadherin 5 promotor. Therefore, possible deletion of MR in leukocytes, as used with the Tie2 promotor, is not likely. Although the specific role of endothelial MR in CVD is unclear, available experimental findings support the hypothesis that during development of CVD, blockade of MR in endothelial cells elicits cardioprotective effects through multiple mechanisms.
Conditional inactivation of MR in VSMCs attenuates age-dependent development of hypertension with improved vascular dysfunction in mice [62]. Similarly, in vascular injury models, attenuation of vascular dysfunction, stiffness, and remodeling were observed in VSMC-specific MR-KO mice [63]. Recently, Gueret et al. [64] showed that specific deletion of MR in VSMCs significantly improved LV function and remodeling after myocardial infarction in association with preserved coronary reserve. The authors also suggested that these effects of VSMC MR inhibition are mediated by improvement of coronary endothelial function via reduction in oxidative stress. Therefore, blocking not only endothelial MR, but also VSMC MR to protect coronary arteries during development of CVD, is important.
Specific role of MR in the pathogenesis of CKD
The kidney consists of many different cells, such as endothelial cells, VSMCs, glomerular mesangial cells, podocytes, proximal tubular cells, distal tubular cells, collecting duct cells, and interstitial fibroblasts. Unfortunately, experimental data with selective deletion or overexpression of MR in these renal cells have been limited. Therefore, the specific role of MR in each renal cell can be speculated based on available data with cell culture and pharmacological experiments.
Specific role of MR in renal vascular and glomerular cells
Acute infusion of neither aldosterone nor an MR antagonist changed renal blood flow in anesthetized rats [2]. However, in vitro studies have shown that aldosterone selectively constricts efferent arterioles through non-genomic pathways [65]. In line with these data, specific deletion of VSMC MR attenuates ciclosporin A-induced renal vasoconstriction and associated acute renal injury [66]. Similarly, specific deletion of MR and Rac1 in VSMCs attenuates acute renal injury after ischemia–reperfusion [67]. These data indicate that specific blockade of renal VSMC MR is a potential therapeutic target for preventing acute renal injury (Fig. 3). However, a previous study showed that specific deletion of MR in endothelial cells did not change ciclosporin A-induced nephrotoxicity [66] and DOCA/salt-induced glomerular injury [59]. Therefore, there is no evidence that indicates a specific role of renal endothelial MR in renal injury.
Specific role of MR in the pathogenesis of CKD. Locally expressed MR in endothelial cells, VSMCs, glomerular mesangial cells, podocytes, proximal tubular cells, distal tubular cells, collecting duct cells, and interstitial fibroblasts is individually involved in the pathogenesis of CKD. AKI acute kidney injury, I/R ischemic-reperfusion injury, LPS lipopolysaccharide
Although MR is abundantly expressed in glomerular mesangial cells [68], specific deletion of mesangial MR has not been successful. This is because of unavailability of the Cre recombinase transgene driven by mesangial-specific genes. However, in vitro studies have shown that aldosterone induces mesangial cell proliferation [68], myofibroblastic transdifferentiation [69], apoptosis [70], and oxidative stress [71], all of which are mediated by locally expressed MR. These data suggest that MR blockade serves as a potential therapeutic approach to mesangial proliferative disease (Fig. 3). Detailed molecular mechanisms responsible for MR-induced mesangial cell injury have been reviewed previously [72].
Fujita and colleagues [73, 74] have proposed that MR is expressed in glomerular podocytes and plays a critical role in the pathogenesis of proteinuria during the development of salt-dependent hypertension and metabolic syndrome. These authors have also demonstrated that MR in podocytes is activated through the Rac1-dependent signaling pathway in a ligand-independent manner [21, 41, 74]. However, Huang et al. [75] showed that specific deletion of MR in podocytes did not affect proteinuria and renal injury in mice with anti-glomerular basement membrane glomerulonephritis. Because eplerenone also did not improve proteinuria and renal injury in this model, MR in podocytes may not play a role in the pathogenesis of glomerulonephritis. Collectively, these studies suggest that selective blockade of MR in podocytes causes a reduction in proteinuria and has a renoprotective effect in subjects with lifestyle-related disease, such as salt-dependent hypertension, diabetes and metabolic syndrome [21] (Fig. 3). However, this blockade does not affect glomerulonephritis, which is an autoimmune disease [75].
Specific role of MR in other renal cells
Huang et al. [75] showed that specific deletion of MR in macrophages leads to protective effects against inflammation and glomerular injury similar to eplerenone in mice with anti-glomerular basement membrane glomerulonephritis. Furthermore, macrophage-specific RacGTPase-KO attenuates renal inflammation induced by lipopolysaccharide [76]. These data suggest that MR in macrophages plays a critical role in the pathophysiology of renal inflammation. Beneficial effects of MR antagonists on renal inflammatory changes may be mediated through its blockade of MR in macrophages, at least in part (Fig. 3).
In cultured proximal tubular cells, aldosterone induces epithelial mesenchymal transition [77] and senescence [78, 79] by activation of MR. Furthermore, aldosterone induces collagen synthesis by activation of MR in cultured renal fibroblasts [80]. These data suggest possible contributions of MR in proximal tubular cells and fibroblasts to progression of tubulointerstitial injury. However, further experiments with selective deletion or overexpression of MR in these renal cells are required to determine the precise mechanism (Fig. 3).
Conclusions
Locally expressed MR in cardiovascular and renal cells is activated by aldosterone-dependent and -independent mechanisms, and individually contributes to the pathogenesis of CVD and CKD. However, beneficial physiological role of MR in cardiovascular and renal non-epithelial cells are not clear. Therefore, understanding the pathophysiological role of MR in all cardiovascular and renal cells is important. Specific MR blockade can be considered by using available steroidal MR antagonists (spironolactone and eplerenone), emerging non-steroidal MR blockers, and/or combination therapies with other therapeutics.
References
Nishiyama A, Kobori H. Independent regulation of renin-angiotensin-aldosterone system in the kidney. Clin Exp Nephrol. 2018;22:1231–9.
Rafiq K, Hitomi H, Nakano D, Nishiyama A. Pathophysiological roles of aldosterone and mineralocorticoid receptor in the kidney. J Pharmacol Sci. 2011;115:1–7.
Nishimura M, Uzu T, Fujii T, Kuroda S, Nakamura S, Inenaga T, et al. Cardiovascular complications in patients with primary aldosteronism. Am J Kidney Dis. 1999;33:261–6.
Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, et al.; Papy Study Participants. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–8.
Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA . 2006;295:2638–45.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone post-acute myocardial infarction heart failure, efficacy survival study, investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
Lin C, Zhang Q, Zhang H, Lin A. Long-term effects of low-dose spironolactone on chronic dialysis patients: a randomized placebo-controlled study. J Clin Hypertens (Greenwich). 2016;18:121–8.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–357.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M. et al.Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253–390.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation . 2017;136:e137–e161.
Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T.Evaluate Study Group Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:944–53.
Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–50.
Vukadinovic D, Lavall D, Vukadinovic AN, Pitt B, Wagenpfeil S, Bohm M. True rate of mineralocorticoid receptor antagonists-related hyperkalemia in placebo-controlled trials: a meta-analysis. Am Heart J. 2017;188:99–108.
Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ. et al.EMPHASIS-HF Investigators Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol. 2013;62:1585–93.
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H. et al.Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884–94.
Hermida RC, Ayala DE, Smolensky MH, Fernandez JR, Mojon A, Portaluppi F. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res. 2016;39:277–92.
Nehme A, Zibara K. Efficiency and specificity of RAAS inhibitors in cardiovascular diseases: how to achieve better end-organ protection? Hypertens Res. 2017;40:903–9.
Karashima S, Yoneda T, Kometani M, Ohe M, Mori S, Sawamura T, et al. Comparison of eplerenone and spironolactone for the treatment of primary aldosteronism. Hypertens Res. 2016;39:133–7.
Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25:514–23.
Shibata S, Ishizawa K, Uchida S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res. 2017;40:221–5.
Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab. 2010;95:1986–90.
Sherajee SJ, Fujita Y, Rafiq K, Nakano D, Mori H, Masaki T, et al. Aldosterone induces vascular insulin resistance by increasing insulin-like growth factor-1 receptor and hybrid receptor. Arterioscler Thromb Vasc Biol. 2012;32:257–63.
Lohmeier TE, Liu B, Hildebrandt DA, Cates AW, Georgakopoulos D, Irwin ED. Global- and renal-specific sympathoinhibition in aldosterone hypertension. Hypertension. 2015;65:1223–30.
Kiyomoto H, Rafiq K, Mostofa M, Nishiyama A. Possible underlying mechanisms responsible for aldosterone and mineralocorticoid receptor-dependent renal injury. J Pharmacol Sci. 2008;108:399–405.
Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92:313–23.
Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–5.
Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–63.
Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17:3438–46.
Sato A, Saruta T. Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens. 2003;16(9 Pt 1):781–8.
Narayan H, Webb DJ. New evidence supporting the use of mineralocorticoid receptor blockers in drug-resistant hypertension. Curr Hypertens Rep. 2016;18:34.
Sato A, Saruta T, Funder JW. Combination therapy with aldosterone blockade and renin-angiotensin inhibitors confers organ protection. Hypertens Res. 2006;29:211–6.
Kitada K, Nakano D, Liu Y, Fujisawa Y, Hitomi H, Shibayama Y, et al. Oxidative stress-induced glomerular mineralocorticoid receptor activation limits the benefit of salt reduction in Dahl salt-sensitive rats. PLoS ONE. 2012;7:e41896.
Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, et al. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension. 2006;47:656–64.
Baker ME, Funder JW, Kattoula SR. Evolution of hormone selectivity in glucocorticoid and mineralocorticoid receptors. J Steroid Biochem Mol Biol. 2013;137:57–70.
Vitellius G, Trabado S, Bouligand J, Delemer B, Lombès M. Pathophysiology of glucocorticoid signaling. Ann Endocrinol (Paris). 2018;79:98–106.
Rafiq K, Nakano D, Ihara G, Hitomi H, Fujisawa Y, Ohashi N, et al. Effects of mineralocorticoid receptor blockade on glucocorticoid-induced renal injury in adrenalectomized rats. J Hypertens. 2011;29:290–8.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6.
Uddin S, Lekmine F, Sharma N, Majchrzak B, Mayer I, Young PR, et al. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon alpha-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J Biol Chem. 2000;275:27634–40.
Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Zhu W, et al. Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circ Res. 1999;84:458–66.
Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–43.
Nagase M, Ayuzawa N, Kawarazaki W, Ishizawa K, Ueda K, Yoshida S, et al. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: role of small GTPase Rac1. Hypertension. 2012;59:500–6.
Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, Perrier R, Soukaseum C, Nguyen Dinh Cat A, et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 2005;111:3025–33.
Favre J, Gao J, Zhang AD, Remy-Jouet I, Ouvrard-Pascaud A, Dautreaux B, et al. Coronary endothelial dysfunction after cardiomyocyte-specific mineralocorticoid receptor overexpression. Am J Physiol Heart Circ Physiol. 2011;300:H2035–2043.
Lother A, Berger S, Gilsbach R, Rosner S, Ecke A, Barreto F, et al. Ablation of mineralocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension. 2011;57:746–54.
Fraccarollo D, Berger S, Galuppo P, Kneitz S, Hein L, Schutz G, et al. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation. 2011;123:400–8.
Yamamuro M, Yoshimura M, Nakayama M, Abe K, Shono M, Suzuki S, et al. Direct effects of aldosterone on cardiomyocytes in the presence of normal and elevated extracellular sodium. Endocrinology. 2006;147:1314–21.
Montes-Cobos E, Li X, Fischer HJ, Sasse A, Kugler S, Didie M, et al. Inducible knock-down of the mineralocorticoid receptor in mice disturbs regulation of the renin-angiotensin-aldosterone system and attenuates heart failure induced by pressure overload. PLoS ONE. 2015;10:e0143954.
Rickard AJ, Morgan J, Bienvenu LA, Fletcher EK, Cranston GA, Shen JZ, et al. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension. 2012;60:1443–50.
Sun JY, Li C, Shen ZX, Zhang WC, Ai TJ, Du LJ, et al. Mineralocorticoid receptor deficiency in macrophages inhibits neointimal hyperplasia and suppresses macrophage inflammation through SGK1-AP1/NF-kappaB pathways. Arterioscler Thromb Vasc Biol. 2016;36:874–85.
Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–43.
Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–64.
Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids. 2014;91:38–45.
Herrada AA, Campino C, Amador CA, Michea LF, Fardella CE, Kalergis AM. Aldosterone as a modulator of immunity: implications in the organ damage. J Hypertens. 2011;29:1684–92.
Ayuzawa N, Nagase M, Ueda K, Nishimoto M, Kawarazaki W, Marumo T, et al. Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension. 2016;67:99–106.
Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Benjamin L, Farman N, Lacolley P, et al. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–63.
Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, et al. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res. 2016;118:935–43.
Mueller KB, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, et al. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension. 2015;66:988–97.
Lother A, Furst D, Bergemann S, Gilsbach R, Grahammer F, Huber TB, et al. Deoxycorticosterone acetate/salt-induced cardiac but not renal injury is mediated by endothelial mineralocorticoid receptors independently from blood pressure. Hypertension. 2016;67:130–8.
Salvador AM, Moss ME, Aronovitz M, Mueller KB, Blanton RM, Jaffe IZ, et al. Endothelial mineralocorticoid receptor contributes to systolic dysfunction induced by pressure overload without modulating cardiac hypertrophy or inflammation. Physiol Rep. 2017;5:e13313.
Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, et al. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension. 2015;66:1159–67.
McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–33.
Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34:355–64.
Gueret A, Harouki N, Favre J, Galmiche G, Nicol L, Henry JP, et al. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension. 2016;67:717–23.
Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F, et al. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol. 2003;14:2255–63.
Amador CA, Bertocchio JP, Andre-Gregoire G, Placier S, Duong Van Huyen JP, El Moghrabi S, et al. Deletion of mineralocorticoid receptors in smooth muscle cells blunts renal vascular resistance following acute cyclosporine administration. Kidney Int. 2016;89:354–62.
Barrera-Chimal J, Andre-Gregoire G, Nguyen Dinh Cat A, Lechner SM, Cau J, Prince S, et al. Benefit of mineralocorticoid receptor antagonism in AKI: role of vascular smooth muscle Rac1. J Am Soc Nephrol. 2017;28:1216–26.
Nishiyama A, Yao L, Fan Y, Kyaw M, Kataoka N, Hashimoto K, et al. Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005;45:710–6.
Diah S, Zhang GX, Nagai Y, Zhang W, Gang L, Kimura S, et al. Aldosterone induces myofibroblastic transdifferentiation and collagen gene expression through the Rho-kinase dependent signaling pathway in rat mesangial cells. Exp Cell Res. 2008;314:3654–62.
Mathew JT, Patni H, Chaudhary AN, Liang W, Gupta A, Chander PN, et al. Aldosterone induces mesangial cell apoptosis both in vivo and in vitro. Am J Physiol Ren Physiol. 2008;295:F73–81.
Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, et al. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol. 2005;16:2906–12.
Nishiyama A, Hitomi H, Rahman A, Kiyomoto H. Drug discovery for overcoming chronic kidney disease (CKD): pharmacological effects of mineralocorticoid-receptor blockers. J Pharmacol Sci. 2009;109:1–6.
Fujita T. Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension. 2010;55:813–8.
Nishimoto M, Fujita T. Renal mechanisms of salt-sensitive hypertension: contribution of two steroid receptor-associated pathways. Am J Physiol Ren Physiol. 2015;308:F377–387.
Huang LL, Nikolic-Paterson DJ, Han Y, Ozols E, Ma FY, Young MJ, et al. Myeloid mineralocorticoid receptor activation contributes to progressive kidney disease. J Am Soc Nephrol. 2014;25:2231–40.
Nagase M, Kurihara H, Aiba A, Young MJ, Sakai T. Deletion of Rac1GTPase in the myeloid lineage protects against inflammation-mediated kidney injury in mice. PLoS ONE. 2016;11:e0150886.
Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am J Physiol Ren Physiol. 2007;293:F723–731.
Fan YY, Kohno M, Hitomi H, Kitada K, Fujisawa Y, Yatabe J, et al. Aldosterone/mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology. 2011;152:680–8.
Kitada K, Nakano D, Hitomi H, Kobori H, Deguchi K, Mori H, et al. Aldosterone induces p21-regulated apoptosis via increased synthesis and secretion of tumour necrosis factor-alpha in human proximal tubular cells. Clin Exp Pharmacol Physiol. 2012;39:858–63.
Nagai Y, Miyata K, Sun GP, Rahman M, Kimura S, Miyatake A, et al. Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension. 2005;46:1039–45.
Acknowledgements
This work was supported by the Naito Foundation, the Hoansha Foundation and Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (18H03191). We thank Ellen Knapp, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AN received honoraria from Boehringer Ingelheim, Daiichi-Sankyo, Mochida, and Taisho-Toyama, as well as research grants from Boehringer Ingelheim, Daiichi-Sankyo, Pfizer, and Taisho-Toyama.
Rights and permissions
About this article
Cite this article
Nishiyama, A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res 42, 293–300 (2019). https://doi.org/10.1038/s41440-018-0158-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0158-6
Keywords
This article is cited by
-
The role of a novel mineralocorticoid receptor antagonist, finerenone, in chronic kidney disease: mechanisms and clinical advances
Clinical and Experimental Nephrology (2024)
-
Efficacy and Safety of Esaxerenone in Hypertensive Patients with Left Ventricular Hypertrophy (ESES-LVH) Study: A Multicenter, Open-Label, Prospective, Interventional Study
Advances in Therapy (2024)
-
Fluid homeostasis induced by sodium-glucose cotransporter 2 inhibitors: novel insight for better cardio-renal outcomes in chronic kidney disease
Hypertension Research (2023)
-
Importance of plasma aldosterone concentrations as a clinical indicator of nocturnal hypertension in primary aldosteronism
Hypertension Research (2023)
-
Sodium butyrate ameliorates deoxycorticosterone acetate/salt-induced hypertension and renal damage by inhibiting the MR/SGK1 pathway
Hypertension Research (2021)