Abstract
Eclampsia is a leading cause of maternal and fetal morbidity and mortality worldwide, and its pathogenesis remains elusive. Our objective was to investigate neuroimaging findings in women who developed neurologic symptoms in severe preeclampsia with or without eclampsia to further understand the relationship between neuroimaging findings and the pathogenesis of eclamptic seizures. This retrospective study included 79 women with severe preeclampsia/eclampsia who underwent brain MRI/CT examination between 2005 and 2017. We analyzed imaging findings, clinical data, and laboratory data in order to compare patients with severe preeclampsia to those with eclampsia and patients with abnormal imaging findings to those with normal CT or MRI. A total of 41 of 79 women were diagnosed with eclampsia, 36 (88.80%) of which had abnormal neuroimaging findings, including cerebral edema (19 cases), infarction (5 cases), cerebral venous thrombosis (5 cases), and cerebral hemorrhage (7 cases). Five patients died of cerebral hemorrhage. Of the 38 cases of severe preeclampsia, 21 (55.26%) cases had abnormal imaging findings, including cerebral edema (20 cases), and 1 case had cerebral hemorrhage. Serum uric acid was significantly higher in patients with abnormal imaging findings than in patients without them (P = 0.004). The imaging findings in women with neurologic symptoms were similar between the severe preeclampsia and eclampsia groups. Our results suggest that eclampsia may not be a diagnosis with a unique pathogenesis; rather, it may be best considered a severe symptom of the intracranial pathophysiology of preeclampsia. We suggest that cranial imaging should be performed early in the management of patients with severe preeclampsia who develop new neurologic symptoms.
Similar content being viewed by others
Introduction
The neurologic symptoms associated with severe preeclampsia (PE) include headaches, dizziness, visual disturbances, impaired consciousness, and seizures. Seizures in the absence of other etiologies are labeled “eclampsia”. Most reports place the incidence of eclampsia at 3/1000 to 9/1000 pregnant women [1, 2] and it is still a leading cause of maternal and fetal mortality worldwide.
The pathophysiology of PE is thought to be primarily related to vascular dysfunction [3], but the actual pathogenesis of eclamptic seizures remains elusive. However, we are now able to visualize and monitor the cerebral impact of hypertensive disorders in pregnancy and eclampsia with MRI or CT scans. Reversible posterior leukoencephalopathy syndrome (RPLS) [4] was a term coined to describe the imaging findings of patients displaying extensive bilateral white-matter abnormalities suggestive of edema in the posterior regions of the cerebral hemispheres. RPLS was initially thought to be a unique cerebral finding in eclampsia, but Topuz [5] described similar imaging findings in pregnancy-induced hypertension. It was noted that these findings were all also found commonly in non-gestational hypertensive encephalopathy.
Older studies on imaging findings in eclampsia are inconsistent, but this consistency may be because many of these studies excluded cases with intracranial hemorrhage or infarction based on the early concept of eclampsia. Therefore, we conducted a retrospective analysis of patients diagnosed with severe preeclampsia who developed new neurologic symptoms with or without eclampsia without excluding patients who had hemorrhage or infarction on neuroimaging to better understand the management of these patients.
Methods
This retrospective study was conducted in the Guangzhou Women and Children’s Medical Center and The Obstetric Critical Care Center of the Third Affiliated Hospital of Guangzhou Medical University from January 2005 to February 2017. We identified patients with eclampsia and those with severe preeclampsia who developed new neurologic symptoms, such as headaches, dizziness, visual disturbances, and mental status changes, who underwent MRI or CT within 3 days of the onset of symptoms. There were 79 patients who fulfilled the criteria, 41 of whom were diagnosed with eclampsia. Maternal characteristics (such as age, parity, and blood pressure), laboratory data, and radiologic findings were collected.
Severe PE was diagnosed based on the diagnostic criteria established by the American College of Obstetrics and Gynecology [6]. Eclampsia was defined as the occurrence of seizures that could not be attributed to other causes in pregnant patients with PE [7].
Magnesium sulfate prophylaxis was used in all patients with severe PE, and some patients were treated with labetalol hydrochloride and calcium channel blockers, such as nifedipine, to control blood pressure before the seizures occurred. All patients received magnesium sulfate by the Pritchard regimen upon diagnosis of severe PE and/or eclampsia.
Subjects were considered to have abnormal imaging if their initial CT or MRI report described cerebral edema, cerebral infarction, cerebral venous thrombosis, or cerebral hemorrhage. If none of the above findings were reported, the subject was categorized as “normal imaging”.
Cerebral edema was generally defined as the presence of imaging findings consistent with excess accumulation of fluid in the intracellular or extracellular spaces of the brain. The CT scan showed hypo-densities in cerebral parenchymal. The MRI showed hyper-intensity lesions on T2 and FLAIR imaging and hypo-intensity on T1 imaging.
SPSS19.0 (SPSS, IBM, New York) was used for statistical analysis. For statistical comparisons, χ2 and Student’s t test were used as appropriate. P values less than or equal to 0.05 were considered statistically significant.
Results
Clinical characteristics of the patients
Of the 79 patients who fulfilled the inclusion criteria, 41 patients (52%) were diagnosed with eclampsia, and 38 (48%) patients were diagnosed with severe PE without eclampsia.
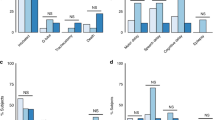
Among the 41 patients in the eclampsia group, 7 (17%) cases occurred postpartum and 5 (12%) cases had no abnormal neuroimaging findings. The white blood cell count and neutrophil percentage were significantly higher in the eclampsia group than in the severe PE group. There was a significant increase in the incidence of placental abruption in the eclampsia group. There was a trend toward higher AST in the eclampsia group. Table 1 shows admission clinical and laboratory data in patients with eclampsia and severe PE. In the severe PE group (i.e., no seizure), all patients were treated with antihypertensive medications (labetalol and/or nifedipine, and/or glyceryl trinitrate, and/or nitroprusside); however, in the eclampsia group, only 12/41 (29%) patients were treated with anti-hypertensive therapy before seizures occurred. All eclampsia patients received anti-hypertensive therapy (labetalol and/or nifedipine, and/or glyceryl trinitrate, and/or nitroprusside) once seizures were identified.
A total of 36 of 41 cases (89%) of eclampsia had abnormal neuroimaging findings, whereas 21 (55%) cases of severe PE had abnormal neuroimaging. Of the eight patients with cerebral hemorrhage, five died—all of whom were in the eclampsia group. Details on the imaging findings are shown in Table 2. Representative MRI/CT examples of each key neuroimaging finding (edema, infarction, hemorrhage, and venous thrombosis) are shown in Figs. 1–4.
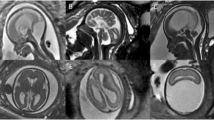
Cerebral edema in CT and MRI in a severe PE patient. Severe PE: A 32-year-old patient, 35+ weeks pregnant, with blurred vision and hypertension. A The arrows show bilateral occipital-temporal hypo-densities in CT; B T1-weighted MR imaging shows hypo-intensity; C T2-weighted MR imaging shows hyper-intensity
Cerebral infarction in MRI in an eclampsia patient. Eclampsia: A 25-year-old patient, 35+ weeks pregnant, with seizure at home. The arrows show abnormal signals in corpus callosum. A T1-weighted MR imaging shows hypo-intensity; B, C T2-weighted MR imaging and T2WI/FLAIR show hyper-intensity; D DWI shows hyper-intensity; E ADC value decreased. All observations suggest cerebral infarction
Table 3 compares the clinical and laboratory characteristics of patients with and without abnormal neuroimaging findings. Uric acid was significantly higher in patients with abnormal imaging than in those with normal imaging, and more patients with abnormal imaging had renal dysfunction and HELLP than patients with normal imaging.
Discussion
Obstetricians and obstetric researchers have typically considered eclampsia to have a unique pathophysiology that develops in a background of PE and use the term for a separate diagnosis within the category of hypertensive disorders of pregnancy. However, in our series, we noted (as did other investigators) [5, 8] that many of the abnormal imaging findings typically associated with eclamptic seizures were found in patients with severe PE who did not experience seizures. This finding raises the possibility that eclampsia may not be a separate disease but rather one of several symptoms (albeit a dramatic one) of the cerebral pathophysiology of PE arising from the barotrauma and inflammatory changes seen in pregnancy-induced hypertension.
In our series, most patients with severe PE and neurologic symptoms had imaging abnormalities, such as cerebral edema, cerebral infarction, and cerebral hemorrhage. Other researchers have reported [8,9,10] similar findings of edema and infarction in women with PE. Matsuda [9] hypothesized that an abnormal cerebral MR may predict the onset of eclampsia in women with severe PE. In their series of 41 PE patients, 11 patients had cerebral edema and 6 of these 11 patients developed seizures after delivery. Mayama [11] reported on 39 patients with neurologic symptoms and PE or eclampsia and found that the clinical and radiologic findings of posterior reversible encephalopathy syndrome were similar between the two groups, supporting the concept that these patient groups have a shared pathophysiologic background.
Hinchey [4] articulated the concept of RPLS to describe the MRI findings of patients displaying extensive bilateral white-matter abnormalities suggestive of edema in the posterior regions of the cerebral hemispheres in 1996. Since then, many researchers have considered PRLS as a unique cerebral pathology of eclampsia [9, 12, 13]. However, some researchers [1, 5, 14] noted that the neuroimaging findings in eclampsia and PE also include cerebral edema and infarction, not just PRLS. As early as 1973, Sheehan reported that autopsy findings in women who died after suffering eclamptic seizures showed cerebral edema, hemorrhage, and infarction [15]. Another observation that supports the concept of eclampsia as a symptom rather than a disease comes from the observation that neuroimaging findings related to hypertensive disorder in pregnancy overlap extensively with non-pregnant patients suffering from hypertensive encephalopathy [16]. In addition, in a rat model of eclampsia, researchers have found biochemical evidence that systemic and neuronal inflammation may decrease the seizure threshold [17,18,19]. Is it possible that the infarction, central venous thrombosis, and hemorrhage seen macroscopically are the source of the increase in local inflammatory responses?
Cerebral insults related to hypertensive disorder in pregnancy can be seen in different imaging findings and require different management approaches, especially for patients with demonstrable abnormal imaging findings on cerebral MRI or CT changes in the brain. Cerebral hemorrhage is not surprisingly associated with a very high mortality rate in patients with PE. In our series, five of seven patients in the eclampsia group with cerebral hemorrhage died. We previously reported that patients with eclampsia and HELLP syndrome were more likely to suffer intracranial hemorrhage and death [20]. Other reports also noted increased mortality in patients with cerebral hemorrhage [5, 21]. Because specific and emergent management is required to reduce morbidity and mortality in patients with cerebral hemorrhage, we believe that early cerebral imaging is highly desirable in patients with a new onset of neurologic symptoms in the setting of PE.
Based on our experience and that of others, we believe that an updated protocol for the evaluation and management of patients who manifest neurologic symptoms with severe PE should be considered (Fig. 5).
Blood pressure elevations were similar in women with severe PE and those who presented with seizure. Because severe hypertension can increase the risk of severe end-organ damage, such as renal failure, placental abruption, and cerebral hemorrhage [22, 23], we suggest that aggressive antihypertensive therapy is very important in pregnant patients with severe hypertension.
One other related observation in our case series was the association between the level of uric acid and a higher risk of abnormal imaging findings. This association was noted in another study as well [21]. As uric acid levels are a marker of oxidative stress, tissue injury, endothelial injury, and renal damage, there is some biological plausibility to the idea that uric acid levels are correlated with the activation of inflammatory cascades and thus are a predictor of more severe complications in patients with severe PE.
Conclusion
The neuroimaging findings in women with neurologic symptoms and severe PE include cerebral edema and hemorrhage. A similar range of abnormalities is seen in women with eclampsia, including cerebral edema, infarction, hemorrhage, and cerebral venous thrombosis. Consideration of the findings from our series and from other reports leads us to suggest that eclampsia may be not a diagnosis with a unique pathogenesis; rather, it should be considered a severe symptom of the intracranial pathophysiology of PE. Due to the high mortality associated with intracranial events in patients with severe PE and eclampsia, we also suggest that neuroimaging should be considered early in the management of patients with severe preeclampsia who develop new neurologic symptoms.
References
Miguil M, Chekairi A. Eclampsia, study of 342 cases. Hypertens Pregnancy. 2008;27:103.
Abalos E,Cuesta C,Carroli G,Qureshi Z,Widmer M,Vogel JP, et al. WHO Multicountry Survey on Maternal and Newborn Health Research Network. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(Suppl 1):14–24.
Tomimatsu T, Mimura K, Endo M, Kumasawa K, Kimura T. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens Res. 2017;40:305–10. https://doi.org/10.1038/hr.2016.152. Epub 2016 Nov 10
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. a reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500.
Topuz S, Kalelioğlu I, Iyibozkurt AC, Akhan S, Has R, Tunaci M, et al. Cranial imaging spectrum in hypertensive disease of pregnancy. Clin Exp Obstet Gynecol. 2008;35:194–7.
American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31.
Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Bethesda, Maryland Am J Obstet Gynecol 2000;183:S1−S22.
Demirtaş O, Gelal F, Vidinli BD, Demirtaş LO, Uluç E, Baloğlu A. Cranial MR imaging with clinical correlation in preeclampsia and eclampsia. Diagn Interv Radiol. 2005;11:189–94.
Matsuda H, Sakaguchi K, Shibasaki T, Takahashi H, Kawakami Y, Furuya K, et al. Cerebral edema on MRI in severe preeclamptic women developing eclampsia. J Perinat Med. 2005;33:199–205.
Osmanağaoğlu MA, Dinç G, Osmanağaoğlu S, Dinç H, Bozkaya H. Comparison of cerebral magnetic resonance and electroencephalogram findings in pre-eclamptic and eclamptic women. Aust N Z J Obstet Gynaecol. 2005;45:384–90.
Mayama M, Uno K, Tano S, Yoshihara M, Ukai M, Kishigami Y,et al. Incidence of posterior reversible encephalopathy syndrome in eclamptic and patients with preeclampsia with neurologic symptoms. Am J Obstet Gynecol. 2016;215:239.e1–e5.
Sengar AR, Gupta RK, Dhanuka AK, Roy R, Das K. MR imaging, MR angiography, and MR spectroscopy of the brain in eclampsia. AJNR Am J Neuroradiol. 1997;18:1485–90.
Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, et al. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013;208:468.e1–e6.
Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190:714–20.
Sheehan H, Lynch J. Pathology of Toxemia of Pregnancy. Baltimore: Williams and Wilkins; 1973.
Price RS, Kasner SE. Hypertension and hypertensive encephalopathy. Handb Clin Neurol. 2014;119:161–7.
Huang Q, Liu L, Hu B, Di X, Brennecke SP, Liu H. Decreased seizure threshold in an eclampsia-like model induced in pregnant rats with lipopolysaccharide and pentylenetetrazol treatments. PLoS ONE. 2014;9:e89333 https://doi.org/10.1371/journal.Pone.0089333
Li X, Han X, Yang J, Bao J, Di X, Zhang G, et al. Magnesium sulfate provides neuroprotection in eclampsia-like seizure model by ameliorating neuroinflammation and brain edema. Mol Neurobiol. 2016 Nov 22. [Epub ahead of print]
Liu L, Han X, Huang Q, Zhu X, Yang J, et al. Increased neuronal seizure activity correlates with excessive systemic inflammation in a rat model of severe preeclampsia. Hypertens Res. 2016;39:701–708. https://doi.org/10.1038/hr.2016.53.
Di XD, Chen DJ, Liu HS, Kuang JL, Huang DJ. Clinical outcomes and characteristics of concurrent eclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Zhonghua Fu Chan Ke Za Zhi. 2010;45:740–4.
Junewar V, Verma R, Sankhwar PL, Garg RK, Singh MK, Malhotra HS, Sharma PK, Parihar A. Neuroimaging features and predictors of outcome. AJNR Am J Neuroradiol. 2014;35:1728–1734.21.
Arulkumaran N, Lightstone L. Severe pre-eclampsia and hypertensive crises. Best Pract Res Clin Obstet Gynaecol. 2013;27:877–84. https://doi.org/10.1016/j.bpobgyn.2013.07.003
Vadhera RB, Simon M. Hypertensive emergencies in pregnancy. Clin Obstet Gynecol. 2014;57:797–805. https://doi.org/10.1097/GRF.0000000000000063
Acknowledgements
We thank Jinying Yang, Yanhong Zhou, Guozheng Zhang, and Xiuyu Pan for their suggestions related to clinical data analysis.
Funding
This case series study was funded through Guangdong Provincial Department of Science and Technology, No. 201213031800335.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study has been reviewed by the hospital ethics committee.
Rights and permissions
About this article
Cite this article
Di, X., Mai, H., Zheng, Z. et al. Neuroimaging findings in women who develop neurologic symptoms in severe preeclampsia with or without eclampsia. Hypertens Res 41, 598–604 (2018). https://doi.org/10.1038/s41440-018-0051-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0051-3
This article is cited by
-
Magnetic resonance spectroscopy and liquid chromatography-mass spectrometry metabolomics study may differentiate pre-eclampsia from gestational hypertension
European Radiology (2023)
-
MRI characteristics of brain edema in preeclampsia/eclampsia patients with posterior reversible encephalopathy syndrome
BMC Pregnancy and Childbirth (2021)
-
Neurological manifestations and neuroimaging presentations in patients with severe preeclampsia: predisposing factors and clinical implications
Neurological Sciences (2019)