Abstract
Recently, a simple device for self-monitoring of daily salt intake was developed, and it is recommended by The Japanese Society of Hypertension. This study aimed to investigate the effects of this device on salt reduction and on lowering blood pressure. In this single blinded, cluster randomized controlled trial, families were randomly assigned to either an intervention or a control group. Participants in both groups attended lectures about salt reduction, but only the intervention group used the self-monitoring device to estimate their daily salt intake. The main outcome measure was the difference in the estimated daily salt intake by spot urine between the two groups after 4 weeks. The secondary outcome was the difference in blood pressure. A total of 105 families (158 participants) were randomized. The mean daily salt intake was 9.04 (SD 1.77) g/day in the control group and 9.37 (SD 2.13) g/day in the intervention group at baseline. After 4 weeks, the mean daily salt intake was 8.97 (SD 1.97) g/day in the control group and 8.60 (SD 2.25) g/day in the intervention group; the mean difference between the two groups was −0.50 g/day (95% confidence interval (CI) −0.95, −0.05; P = 0.030). The mean difference in systolic blood pressure was −4.4 mm Hg (95% CI −8.7, −0.1; P = 0.044). This is the first randomized controlled trial to demonstrate the effectiveness of a device for self-monitoring of salt intake with a significant reduction in daily salt intake and systolic blood pressure.
Similar content being viewed by others
Introduction
Self-monitoring, which has a strong theoretical foundation for behavior change, has been proven to be an effective strategy for various health behavior changes, including smoking cessation, diet modification, and physical activity [1, 2]. Salt reduction is one of the top three priority actions for the prevention of non-communicable diseases recommended by the World Health Organization [3], and it has been reported that accurately evaluating one’s own daily salt intake effectively leads to appropriate guidance and judgment as to whether the salt restriction target has been attained [4, 5]. The most accurate way to estimate daily salt intake is to measure urinary sodium excretion by 24-h urine sampling. However, this technique is difficult to implement because it imposes a large burden on patients [6, 7]. In addition, it does not reflect long-term salt intake [8]. Several other methods for estimating daily salt intake have been reported, such as spot urine, nighttime urine, and the second urine after waking; however, the implementation of repeated urinary tests at medical institutions is not feasible [4].
Recently, a simple, self-monitoring device that estimates salt intake by analyzing data from overnight urine using a preinstalled calculation formula was developed [9]. Patients can use this device at home to determine their estimated daily salt intake. Several studies have reported the validity of using this device to monitor estimated daily salt intake [10,11,12]. Although it has been recommended as a useful tool by the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension [4], its effectiveness for salt reduction and lowering blood pressure (BP) has been suggested by only pretest–post-test design studies with weak evidence [9, 13, 14]. Accordingly, the aim of the current study was to investigate the effects of this device on salt reduction and lowering BP using a randomized controlled trial study design.
Methods
The study protocol has been registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR: 000023788). We followed the CONSORT 2010 statement: extension to cluster randomized trials [15].
Study design and participants
The study was a single blinded, family-based, cluster randomized controlled trial. It was conducted in collaboration with the following local organizations/municipalities in the southern part of Fukushima Prefecture, Japan: the Japan Agricultural Cooperative Yumeminami Branch, Nishigo Village, Shirakawa Kosei General Hospital, Shirakawa City, and Sukagawa City. We recruited participants aged 20 years old or older. The trial was held at the Japan Agricultural Cooperative Yumeminami Branch and in Nishigo Village between September and October 2016, in Shirakawa Kosei General Hospital between November and December 2016, in Shirakawa City between January and February 2017, and in Sukagawa City between March and April 2017.
Randomization
Families (clusters) were randomly assigned (1:1) to either an intervention or a control group. Simple randomization was performed with the use of a centralized computer.
Blinding
Participants were not blinded because the trial compared nonpharmacological interventions that could not be masked. The estimated daily salt intake by spot urine at baseline and 4 weeks after the intervention was measured by blinded laboratory technicians.
Intervention
Participants in both the intervention and control groups attended lectures about salt reduction. The content of the lectures included “Salt intake and health longevity” by a general physician (T.T.) and “Tips for effective salt reduction” by a registered dietitian (M.I.). Each lecture lasted 30 min. We handed out leaflets about salt reduction to the participants of both groups.
In the intervention group, participants used the self-monitoring device to estimate their daily salt intake, and they recorded their results for 4 weeks. The device was developed by Kono ME Laboratory, Kanagawa, Japan [9]. This device consists of a 1-l urine cup and an electrical device with volume and conductivity sensors (Fig. 1). The volume sensor, which consists of 50 small resistant chips, measures overnight urine volume in the cup. Since the conductivity sensor measurement can be overestimated by other electrolytes, such as potassium, the sodium chloride concentration was measured and then adjusted using a correlation formula as the value between the values obtained with the ion electrode method and the conductivity method. These values were then integrated into estimated salt excretion in the overnight urine sample. With the assumption of an 8-h sleep period, the self-monitoring device estimates 24-h salt excretion using the following formula: 24-h salt excretion (g/day) = 1.95x + 4.5, where X is the estimated salt excretion in the overnight urine sample. In the intervention group, participants voided completely and discarded their urine before going to bed. Then, they collected all urine anytime they used the restroom throughout the night in the urine cup and measured their estimated daily salt intake using the device.
Main outcome measure
Our primary outcome was the difference in the daily salt intake estimated by spot urine between the intervention and control groups 4 weeks after the intervention.
For the estimation of daily salt intake by spot urine, we applied the equation recommended by the Japanese Society of Hypertension [4], which is a simple method of obtaining a valid approximation of measured 24-h urinary sodium excretion [16]. The formula for estimated daily salt intake by spot urine was as follows: 24-h salt excretion (g/day) = 0.0585 × 21.98 × ((NaS/CrS) × Pr.UCr24)0.392, where NaS is the sodium concentration in spot urine (mEq/L), CrS is the creatinine concentration in spot urine (mg/L), Pr.UCr24 is the estimated 24-h urinary creatinine excretion (mg/day), and Pr.UCr24 = −2.04 × age + 14.89 × body weight (kg) + 16.14 × height (cm) −2244.45.
The secondary outcome was the difference in BP between the two groups 4 weeks after the intervention. For the BP measurement, trained physicians (T.T. and S.S.) measured the BP of each participant twice, with a minimum of 5 min rest between measurements, using a validated automatic BP monitor (Omron HEM-1010; Omron Healthcare, Kyoto, Japan) [17]. Duplicate measurements were averaged.
Estimation of urinary potassium excretion
As briefly mentioned above, the value of 24-h salt excretion by the self-monitoring device can be overestimated when potassium excretion is high. To assess the influence of high potassium excretion, we estimated potassium excretion by the spot urine method with the following formula: 24-h potassium excretion (mmol/day) = 7.59 × ((KS/CrS) × Pr.UCr24)0.431, where KS is the potassium concentration in spot urine (mEq/L) [16].
Sample size calculation
From the results of our prior trial for salt reduction, the mean daily salt intake of the participants was 9.8 g/day (SD 2.1) [18]. Seventy-five participants per group were required to detect a difference of 1 g of daily salt intake between the two groups, with the following assumptions: one family member per participant, intracluster correlation of 0.01, alpha error of 0.05, and power of 0.80 [19].
Statistical analysis
Our main analysis was based on intention to treat. We tested the effects of the intervention on the outcomes using linear mixed models with robust variance estimates, with participants nested within family units and families nested within the local organizations/municipalities. The model assumed an independent covariance structure between the random effects. Because our calculated sample size was not large, we planned to adjust for sex, age, body mass index, use of antihypertensives, chronic kidney disease (CKD), and pre-intervention daily salt intake estimated by spot urine in the analysis of the primary outcome. In the analysis of the secondary outcome, we adjusted for sex, age, body mass index, use of antihypertensives, and pre-intervention BP. To account for missing data on continuous outcomes, we carried out 20 imputations by the Markov Chain Monte Carlo method.
Furthermore, we conducted sensitivity analysis of the full analysis set that included only participants who completed both pre-intervention and post-intervention urinalysis to examine the robustness of our conclusions in the primary analysis.
A two-sided P value < 0.05 was considered statistically significant. Statistical analyses were performed using a commercial software package (STATA, version 14.2 SE; StataCorp LP, College Station, TX).
Ethical considerations
This study was performed according to the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Japanese Ministry of Health, Labour and Welfare. The study protocol was approved by the Ethics Committee of Fukushima Medical University (2780). Written consent was obtained from all participants. All participants were informed that they were free to discontinue their participation at any time without penalty. To ensure equality between the intervention and control groups, participants in the control group used the device after the trial for 4 weeks.
Results
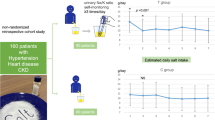
We recruited 158 participants from 105 families. During the trial, four participants in each group did not attend the follow-up measurements. Figure 2 shows the trial profile.
Table 1 shows the baseline characteristics of the participants in each group. The two groups were generally balanced in most parameters; however, participants in the intervention group were younger, had more frequent use of antihypertensives, and had higher estimated daily salt intake at baseline than participants in the control group.
Table 2 shows daily salt intake estimated by spot urine in each group. The mean daily salt intake was 9.04 (SD 1.77) g/day in the control group and 9.37 (SD 2.13) g/day in the intervention group at baseline. Four weeks after the intervention, the mean salt intake was 8.97 (SD 1.97) g/day in the control group and 8.60 (SD 2.25) g/day in the intervention group; the mean difference between the two groups was −0.50 g/day (95% confidence interval (CI) −0.95, −0.05; P = 0.030). The mean difference between the two groups was −4.4 mm Hg (95% CI −8.7, −0.1; P = 0.044) in systolic BP (SBP) and −0.5 mm Hg (95% CI −4.2, −3.2; P = 0.793) in diastolic BP (DBP). The difference in daily salt intake estimated by the spot urine method did not show a significant correlation with the difference in BP (P = 0.94 for SBP and P = 0.99 for DBP).
Sensitivity analysis
The results of sensitivity analysis of the full analysis set were very similar to those of the main analysis (Table 2). The mean difference between the two groups in daily salt intake was −0.51 g/day (95% CI −0.86, −0.16; P = 0.005). The mean difference between the two groups was −5.8 mm Hg (95% CI −7.7, −4.0; P < 0.001) in SBP and −0.2 mmHg (95% CI −4.4, −4.1; P = 0.937) in DBP.
Changes in daily salt intake estimated by the self-monitoring device
In the intervention group, the mean recording rate of the results of the device was 89.5%. The values of daily salt intake estimated by the device were as follows: median 9.44, mean 9.30, SD 1.71 g/day in the first week; median 9.52, mean 9.50, SD 1.65 g/day in the second week; median 9.11, mean 9.50, SD 1.78 g/day in the third week; and median 9.22, mean 9.27, SD 1.76 g/day in the last week (Fig. 3). Although the mean difference between the first and the last week was only −0.02 (SD 1.44, min −4.03, max 4.05) g/day, the difference between the median values was −0.15 g/day.
Potassium excretion estimated by the spot urine method
At baseline, the mean 24-h potassium excretion in the control and intervention group was 38.6 (SD 7.73) mmol/day and 39.0 (SD 7.66) mmol/day, respectively. Four weeks after the intervention, it was 38.1 (SD 7.46) mmol/day and 37.3 (8.43) mmol/day, respectively, for the control and intervention group.
Correlation between salt intake estimated by the device and spot urine
As reported in previous studies [9, 13], the correlation between salt intake estimated by the device and spot urine was moderate (r = 0.57, P < 0.01; Fig. 4).
Discussion
As in studies of other health behavior changes, our results suggested the effectiveness of self-monitoring in salt reduction. We found a significant difference in daily salt intake and SBP between the intervention and control groups. Although the value of daily salt intake estimated by the device could be less accurate than other evaluation methods used in hospitals, it is convenient and can be performed by patients themselves at home [4]. Thus, as recommended by The Japanese Society of Hypertension [4], the device could be an useful tool for salt reduction. While the daily salt intake estimated by the spot urine method decreased in the intervention group, there was little change in daily salt intake estimated by the self-monitoring device. Because the lecture on “Tips for effective salt reduction” given by a registered dietitian (M.I.) included the recommendation of increasing fruit and vegetable intake, we hypothesized that participants in the intervention group increased potassium intake, leading to overestimation of the value of 24-h salt excretion by the self-monitoring device. However, potassium excretion decreased in the intervention group 4 weeks after the intervention. One possible explanation was that the participants in the intervention group might have avoided pickles, which have a high amount of salt, according to the recommendation in the lecture by the dietitian. The dissociation in the values of estimated salt intake between the spot urine method and the self-monitoring device could be caused by other factors, such as excretion of cations other than potassium, temperature, perspiration, and sleeping time. The relationship between salt intake and hemodynamics has been evaluated in many previous studies [20, 21]. Although a previous meta-analysis of randomized trials on salt reduction showed that a 1 g/day reduction in salt intake led to an ~1 mmHg reduction in SBP [20], using the self-monitoring device led to a −4.4 mmHg reduction in SBP in the present study, with a relatively small reduction of −0.50 g/day in daily salt intake estimated by spot urine. Furthermore, the difference in daily salt intake estimated by spot urine did not show a significant correlation with the difference in BP. The dissociation between the reduction in SBP and salt intake could be explained by the fact that salt intake estimated by spot urine reflects the salt intake of only 1 day and does not reflect longer-term salt intake. In addition, it is possible that participants in the intervention group gained a high awareness of BP control by using the device and changed their behavior through exercise, diet, medication, or other means. If such behavior change did occur, it could be regarded as an indirect effect of using the device. Unfortunately, we did not prohibit participants from making such behavior changes nor did we collect information about them. Two previous reports investigated the effects of the device on salt reduction with a pretest–post-test design. In the study by Yamasue et al. [9], 46 adults measured their daily salt intake with the device along with their BP. In the observation period of 21–66 days with a mean of 4 weeks, the mean daily salt intake estimated by the device decreased significantly from 11.06 (SD 1.81) g/day to 10.47 (SD 1.76) g/day, and SBP decreased from 137 (SD 17) mmHg to 133 (SD 16) mmHg. In the other study by Ohta et al. [13], 59 adults measured their daily salt intake with the device. In the observation period of 30 days, the mean daily salt intake estimated by the device decreased from 8.52 (SD 1.63) g/day for the first 10 days to 8.31 (SD 1.54) g/day for the last 10 days (P < 0.05). Our results showed a similar tendency to these two previous studies. However, studies with a pretest–post-test design are generally regarded as weak because there is no comparison group, making the results especially vulnerable to threats to internal validity [22]. Thus, these two previous studies could not distinguish whether the significant reductions in daily salt intake and SBP were caused by use of the device. To the best of our knowledge, this is the first randomized controlled trial to investigate the effects of a simple device for self-monitoring of daily salt intake on salt reduction.
Limitations
Several limitations of this study should be acknowledged. First, our trial was conducted in the southern part of Fukushima Prefecture, a relatively rural area of Japan. Accordingly, the external validity of our findings in other areas, including foreign countries, should be further investigated. Second, daily salt intake values for statistical analysis were estimated using spot urine sampling. Although this approach is less reliable than measurements using 24-h collected urine, we chose the spot urine method because of its greater convenience compared with 24-h collected urine sampling. Third, the spot urine method could be affected by the timing of urine collection. However, we did not specify the time of urine collection because this approach was thought to be more convenient for the study participants. Finally, our trial showed statistically significant results 4 weeks after the intervention. However, it has been reported that maintaining the effects of salt reduction intervention is difficult over the long term [23]. Thus, the long-term effectiveness of our intervention should be further investigated.
In conclusion, this randomized controlled trial suggested the effectiveness of a self-monitoring device in significantly reducing daily salt intake and SBP. Measuring estimated daily salt intake using the device at home could be a useful guide for salt reduction.
References
Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111:92–102.
Sanders JP, Loveday A, Pearson N, Edwardson C, Yates T, Biddle SJ, Esliger DW. Devices for self-monitoring sedentary time or physical activity: a scoping review. J Med Internet Res. 2016;18:e90 https://doi.org/10.2196/jmir.5373
Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, Baugh V, Bekedam H, Billo N, Casswell S, Cecchini M, Colagiuri R, Colagiuri S, Collins T, Ebrahim S, Engelgau M, Galea G, Gaziano T, Geneau R, Haines A, Hospedales J, Jha P, Keeling A, Leeder S, Lincoln P, McKee M, Mackay J, Magnusson R, Moodie R, Mwatsama M, Nishtar S, Norrving B, Patterson D, Piot P, Ralston J, Rani M, Reddy KS, Sassi F, Sheron N, Stuckler D, Suh I, Torode J, Varghese C, Watt J, Lancet NCD Action Group; NCD Alliance. Priority actions for the non-communicable disease crisis. Lancet. 2011;377:1438–47.
Kawano Y, Tsuchihashi T, Matsuura H, Ando K, Fujita T, Ueshima H, Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension. Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension: (2) assessment of salt intake in the management of hypertension. Hypertens Res. 2007;30:887–93.
Yasutake K, Miyoshi E, Kajiyama T, Umeki Y, Misumi Y, Horita N, Murata Y, Ohe K, Enjoji M, Tsuchihashi T. Comparison of a salt check sheet with 24-h urinary salt excretion measurement in local residents. Hypertens Res. 2016;39:879–85.
Ohta Y, Tsuchihashi T, Onaka U, Miyata E. Long-term compliance of salt restriction and blood pressure control status in hypertensive outpatients. Clin Exp Hypertens. 2010;32:234–8.
Ohta Y, Tsuchihashi T, Ueno M, Kajioka T, Onaka U, Tominaga M, Eto K. Relationship between the awareness of salt restriction and the actual salt intake in hypertensive patients. Hypertens Res. 2004;27:243–6.
Sakaki M, Tsuchihashi T, Arakawa K, Fukui H, Kameda W, Tominaga M. Long-term variability of urinary salt excretion and blood pressure in hypertensive patients. Hypertens Res. 2014;37:939–43.
Yamasue K, Tochikubo O, Kono E, Maeda H. Self-monitoring of home blood pressure with estimation of daily salt intake using a new electrical device. J Hum Hypertens. 2006;20:593–8.
Yasutake K, Horita N, Murata Y, Koyama S, Enjoji M, Tsuchihashi T. Estimated urinary salt excretion by a self-monitoring device is applicable to education of salt restriction. Hypertens Res. 2015;38:143–8.
Yasutake K, Horita N, Umeki Y, Misumi Y, Murata Y, Kajiyama T, Ogimoto I, Tsuchihashi T, Enjoji M. Self-management of salt intake: clinical significance of urinary salt excretion estimated using a self-monitoring device. Hypertens Res. 2016;39:127–32.
Yasutake K, Sawano K, Shono N, Tsuchihashi T. Validation of a self-monitoring device for estimating 24-hour urinary salt excretion. Asia Pac J Clin Nutr. 2013;22:25–31.
Ohta Y, Tsuchihashi T, Miyata E, Onaka U. Usefulness of self-monitoring of urinary salt excretion in hypertensive patients. Clin Exp Hypertens. 2009;31:690–7.
Yasutake K, Sawano K, Yamaguchi S, Sakai H, Amadera H, Tsuchihashi T. Self-monitoring urinary salt excretion in adults: A novel education program for restricting dietary salt intake. Exp Ther Med. 2011;2:615–8.
Campbell MK, Piaggio G, Elbourne DR, Altman DG, Group C. Consort 2010 statement: extension to cluster randomised trials. Br Med J. 2012;345:e5661 https://doi.org/10.1136/bmj.e5661
Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103.
Takahashi H, Yoshika M, Yokoi T. Validation of home blood pressure-monitoring devices, Omron HEM-1020 and Omron i-Q132 (HEM-1010-E), according to the European Society of Hypertension International Protocol. Blood Press Monit. 2011;16:203–7.
Takada T, Imamoto M, Fukuma S, Yamamoto Y, Sasaki S, Uchida M, Miura Y, Shimizu S, Nihata K, Fukuhara S. Effect of cooking classes for housewives on salt reduction in family members: a cluster randomized controlled trial. Public Health. 2016;140:144–50.
van Breukelen GJ, Candel MJ. Calculating sample sizes for cluster randomized trials: we can keep it simple and efficient! J Clin Epidemiol. 2012;65:1212–8.
He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. Br Med J. 2013;346:f1325 https://doi.org/10.1136/bmj.f1325
Han W, Han X, Sun N, Chen Y, Jiang S, Li M. Relationships between urinary electrolytes excretion and central hemodynamics, and arterial stiffness in hypertensive patients. Hypertens Res. 2017;40:746–51.
Portney LG, Watkins MP, Foundations of Clinical Research. Applications to Practice. Philadelphia, FA, USA: Davis Company; 2015. p. 193–234..
Adler AJ, Taylor F, Martin N, Gottlieb S, Taylor RS, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;12:CD009217 https://doi.org/10.1002/14651858.CD009217.pub3
Acknowledgements
We thank the Japan Agricultural Cooperative Yumeminami Branch, Nishigo Village, Shirakawa City, and Sukagawa City for supporting the project; Sayuri Yamada, Hiromi Saisu, and Nozomi Aita in the Department of Nutrition at Shirakawa Kosei Hospital for lecture assistance; Kaori Omata of Shirakawa Kosei Hospital for managing the project; and all participants in this study.
Funding:
T.T. received a grant from JSPS KAKENHI (Grant Number JP16K19255). No funding was received by the remaining authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takada, T., Imamoto, M., Sasaki, S. et al. Effects of self-monitoring of daily salt intake estimated by a simple electrical device for salt reduction: a cluster randomized trial. Hypertens Res 41, 524–530 (2018). https://doi.org/10.1038/s41440-018-0046-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0046-0
This article is cited by
-
Technology-supported behavior change interventions for reducing sodium intake in adults: a systematic review and meta-analysis
npj Digital Medicine (2024)
-
Dietary salt intake in Japan - past, present, and future
Hypertension Research (2022)
-
Practical and personal education of dietary therapy in hypertensive patients
Hypertension Research (2020)