Abstract
Recent studies suggest that L-type calcium channel blockers (CCBs) contribute to reducing blood pressure (BP) variability. We investigated whether inhibition of the N-type calcium channel has an additional effect on BP variability by comparing the effect of L-type and L/N-type CCBs on home BP variability in elderly hypertensive patients. Twenty-six hypertensive patients (≥65 years) were subjected to repeated changes with the administration of amlodipine (L-type CCB) and cilnidipine (L/N-type CCB) every 2 months. They measured the home BP in the morning and evening, and the coefficient of variation (CV) was calculated. We measured the brachial-ankle pulse wave velocity (baPWV) and urinary catecholamine excretion as an index of the arterial stiffness and sympathetic nerve activity, respectively. There was no difference in the effect of both drugs on the CV in the morning and evening, while amlodipine was associated with a modestly higher pulse rate and lower BP than cilnidipine. By comparing individual patient data for the CV with each drug, we found that higher urinary catecholamine excretion was associated with the effectiveness of cilnidipine over amlodipine in the BP variability in the morning, which was not the case in the evening. In contrast, lower baPWV was associated with the effectiveness of amlodipine over cilnidipine on BP variability in the evening. Lower baPWV was also associated with lower BP variability in the evening. Cilnidipine has a similar capacity as amlodipine in reducing home BP variability, but the underlying mechanisms in reducing BP variability may differ.
Similar content being viewed by others
Introduction
Recent studies have suggested that increased blood pressure (BP) variability predicts a poor prognosis for hypertensive patients independent of the elevated absolute value of BP [1,2,3,4,5,6,7,8,9]. While the development of antihypertensive drugs in the last few decades has made it possible to control the average BP in the majority of hypertensive patients, an optimal strategy to decrease BP variability in these patients remains under investigation. One possible strategy to decrease BP variability is to use a calcium channel blocker (CCB) instead of the other classes of drugs. [5, 8, 10,11,12,13] Rothwell et al. analysed the data of the ASCOT–BPLA trial in which amlodipine-based regimens were superior to atenolol-based regimens in reducing cardiovascular diseases and found that amlodipine reduced within-visit variability, as well as variability in 24-h ambulatory BP monitoring (ABPM) compared to atenolol [11]. It was also reported that the combination of an ARB with a CCB was superior to that of an ARB with diuretics in reducing home BP variability in patients with type II diabetes [9] and essential hypertension [8]. Given the different mechanisms to reduce BP among CCBs, β-blockers, and diuretics, it is suggested that the capacity of CCBs to reduce vascular resistance by blocking the L-type Ca channel contributes to the reduction in the BP variability.
Cilnidipine has a similar capacity to reduce BP compared with the most commonly prescribed CCB, amlodipine, but there is a distinct difference between these drugs because of the additional capacity of cilnidipine to block N-type calcium channels [14,15,16]. Some studies demonstrated that cilnidipine has protective effects on baroreflex sensitivity [17] or vascular function [18] through suppression of the sympathetic nerve activity with inhibition of N-type calcium channels. As fluctuations in sympathetic nerve activity are associated with BP variability, we hypothesised that inhibition of N-type calcium channels by cilnidipine would have additional effects on BP variability. To clarify the hypothesis, elderly hypertensive patients were subjected to intensive home BP monitoring and the change in BP variability was assessed during repeated change-over from amlodipine to cilnidipine. We also investigated whether arterial stiffness, assessed by the brachial-ankle pulse wave velocity (baPWV), and urinary catecholamine excretion as an index of the sympathetic nerve activity influenced the effects of the two drugs on BP variability.
Methods
Study subjects
We performed a hospital-based prospective study between December 2014 and January 2016 at Osaka University medical hospital. We recruited patients with essential hypertension aged 65 or older who received amlodipine and other antihypertensive drugs, if any. Patients were excluded if their prescription for antihypertensive drugs except the dose of amlodipine had changed within 6 months before recruitment or if they had a medical history of myocardial infraction or stroke within 6 months, heart failure (the New York Heart Association class III or IV), arterial fibrillation or severe liver dysfunction. We enroled 31 patients, and 26 of them completed the study protocol (Fig. 1a). The ethics committee in Osaka university hospital approved the study protocol, and all patients provided written informed consent (Approval number; 13423-2). We performed the study according to the ethical declaration of Helsinki and Good Clinical Practice standards.
Study design
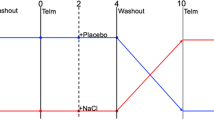
In this repeated change-over study, amlodipine and cilnidipine were sequentially given to the same patient as shown in Fig. 1b. After confirmation of eligibility and providing written informed consent, patients who took amlodipine started to measure their home BP as instructed and were requested to visit the hospital at an interval of 2 months (the first amlodipine period). At the next visit, 2.5 mg, 5 mg or 10 mg of amlodipine were changed to 5 mg, 10 mg or 20 mg of cilnidipine, respectively, because there was an equivalent antihypertensive effect between 5 mg of amlodipine and 10 mg of cilnidipine in a previous randomised study [19] (the first cilnidipine period). Thereafter, physicians sequentially changed the prescription of the test drug from cilnidipine to amlodipine and from amlodipine to cilnidipine in the same fashion at an interval of 2 months (the second cilnidipine period and second amlodipine period). At each visit, physicians measured office BP in a seated position, blood and urinary samples were collected, and a medical interview was performed to confirm correct measurement of home BP and high compliance with the medication. Antihypertensive medications, except the test drug, were unchanged throughout the study. We excluded the data of one subject from the analysis because a physician needed to increase the dose of the test drugs to treat uncontrolled BP.
Home BP measurements
During the study period, the patients measured home BP and pulse rate (PR) twice a day in the early morning and evening before sleep with an electronic sphygmomanometer that transmits the data wirelessly to database in a secure website to which access is limited to a physician in the hospital (HEM-7251G-HP, Omron healthcare Co, Kyoto, Japan) [20]. The patients measured their home BP at least three times in the sitting position after 2 mins of rest according to the guidelines of the Japanese Society of Hypertension. Considering the carry over effects of the test drugs, we used the data from 28 days during the latter half in each period (Fig. 1b). We used the averaged BP and PR among three measurements to obtain the BP and PR at each time-point, respectively. We calculated the coefficient of variation (CV) to assess the variability in the BP and PR with CV = Standard deviation/mean value.
Pulse wave velocity
The measurements of the brachial-ankle pulse wave velocity (baPWV) with the ankle brachial pressure index (ABI) were performed in the morning at the visit after the second amlodipine period with the volume-plethysmographic device (VP-2000; Omron Healthcare, Japan). The device evaluates both the brachial and ankle BP, as well as the pulse waves of the carotid and both femoral arteries at the same time in the supine positon [21, 22].
Biochemical measurements
The estimated glomerular filtration rate (eGFR) was calculated using the Japanese glomerular filtration rate equation based on serum creatinine, gender and age [23]. The urine samples in the morning for catecholamine were immediately frozen after collection, and the measurement was performed in SRL (Tokyo, Japan). The urinary catecholamine per creatinine was calculated.
Log amlodipine/cilnidipine CV
We divided patients into the smaller CV with amlodipine group and smaller CV with cilnidipine group based on the comparison of BP variability between the total amlodipine and cilnidipine periods. We calculated the log ratio of the CV of systolic BP during the amlodipine periods to the CV during the cilnidipine period of the same patient (log2 (amlodipine CV/cilnidipine CV) = log A/C CV). If the log ratio exceeded 0, we classified the patient as the smaller CV with cilnidipine group; if the ratio was less than 0, we classified the patient as the smaller CV with amlodipine group.
Statistical analysis
As shown in Fig. 1b, we planned a study of a continuous response variable from matched pairs of study subjects. Previous researches indicate that the difference in the response of matched pairs is normally distributed with standard deviation 8 [24]. If the true difference in the mean response of matched pairs is 5, we will need to study 22 pairs of subjects to be able to reject the null hypothesis that this response difference is 0 with power 0.8. The Type I error probability associated with this test of this null hypothesis is 0.05. The sample size was calculated with Power and Sample Size Calculation ver 3.1.2 software (Vanderbilt University Department of Biostatistics, TN, USA).
All data were analysed with JMP Pro ver 12.0.1 software (SAS Institute Inc., Cary, NC, USA). Values are shown as the mean ± standard deviation, median (interquartile range), or percentage. Paired t-tests were used to analyse the changes between the two CCBs in the average value of the BP and PR, CV in systolic BP (SBP) and PR. Student’s t-test and Fisher’s exact test were employed to examine the difference in several factors between two groups. Pearson’s correlation coefficient was used to examine univariate correlations. Stepwise regression analysis was used to determine variables related to log A/C CV. The logarithm was calculated to normal distribution. P < 0.05 was considered statistically significant.
Results
Comparison of values and variabilities of BP and PR between the amlodipine and cilnidipine treatments
In 26 subjects, who completed the study, the correct BP measurement of all the patients were confirmed by raw data with measurement times and an interview at each visit. Table 1 shows the baseline characteristics of the study subjects. The average office BP was less than 140/90 mm Hg, and the BP of the patients was well controlled throughout the study period. As shown in Table 2, systolic and diastolic BP were modestly, but significantly, higher in both the morning and evening in the cilnidipine periods compared to the amlodipine periods. In contrast, PR was modestly lower in the morning and evening in the cilnidipine periods compared to the amlodipine periods. Regarding variabilities in the BP and PR, we did not observe any difference between the cilnidipine and amlodipine periods in the CV of systolic BP, diastolic BP and PR in the morning and evening.
Factors associated with the effectiveness of amlodipine and cilnidipine on BP variability
As we found no difference in the variabilities in the BP between the amlodipine and cilnidipine periods in all patients, we then divided patients into the smaller CV with amlodipine and smaller CV with cilnidipine groups based on the CV in systolic BP to determine the factors that are associated with the effects of each drug on BP variability. We classified a patient into the smaller CV with cilnidipine group if the CV in the amlodipine periods was higher than that in the cilnidipine period (log A/C CV > 0) and the smaller CV with amlodipine group if log A/C CV was less than 0, as described in the Methods section. Of 26 patients, 13 and 15 patients were classified into the smaller CV with amlodipine group in the morning (Table 3a) and evening (Table 3b), respectively. The background characteristics were similar between two groups. There were no differences in systolic and diastolic BP between the two groups (data not shown). We found that the urinary excretion of total catecholamines, adrenaline and dopamine were significantly higher in the smaller CV with cilnidipine group than in the smaller CV with amlodipine group in the morning. We observe this difference during amlodipine treatments, while not during cilnidipine treatments. There was no significant difference in the baPWV between the groups in the morning (Table 3a). In contrast, baPWV was significantly lower in the smaller CV with amlodipine group than in the smaller CV with cilnidipine group in the evening, while there was no significant difference in the urinary catecholamine excretion between the two groups in both the amlodipine and cilnidipine periods (Table 3b).
Figure 2a shows that the urinary adrenaline excretion was positively correlated with log A/C CV in the morning, suggesting that cilnidipine was more effective than amlodipine in reducing BP variability in patients with higher urinary adrenaline excretion. There was no correlation between the urinary adrenaline excretion and log A/C CV in the evening (Fig. 2a). In contrast, baPWV was positively correlated with log A/C CV in the evening, which was not the case in the morning (Fig. 2b). We confirmed these correlations after adjusting for potential cofounding variables by stepwise regression analysis. As shown in Table 4, we observed a significant correlation between log A/C CV in the morning and urinary adrenaline excretion after adjusting for sex and baPWV (model 1) and for sex and age (model 2). Similarly, we observed a significant correlation between log A/C CV in the evening and baPWV after adjusting for sex and urinary adrenaline excretion (model 3), as well as for sex and age (model 4). We found that the baPWV was positively correlated with CV in the amlodipine period in the evening, which was not the case in the morning (evening: correlation coefficient (r) = 0.49, p = 0.015; morning: r = 0.036, p = 0.87).
a Correlation between Log A/C CV and urinary adrenaline excretion. b Correlation between Log A/C CV and baPWV. Pearson’s correlation coefficient was used to examine correlations. log A/C CV log2 (amlodipine CV/cilnidipine CV), r Pearson correlation coefficient, CV coefficient of variation, baPWV brachial-ankle pulse wave velocity
Discussion
In this study, we found that there was no difference in the effect of cilnidipine and amlodipine on home BP variability. We also performed an additional analysis to investigate the factors associated with the difference of both CCBs in BP variability for individual patients. We found that high urinary catecholamine excretion was associated with the effectiveness of cilnidipine over amlodipine in reducing morning BP variability, which was not the case in the evening. In contrast, low arterial stiffness was associated with the effectiveness of amlodipine compared to cilnidipine in reducing BP variability in the evening, which was not the case in the morning, suggesting that amlodipine is more effective in reducing evening BP variability in patients with lower arterial stiffness. Several studies suggested that CCBs are superior to other types of antihypertensive drugs in reducing home BP variabilities [9]. Asayama et al. recently reported the opposing results in which there was no difference among the effects of CCBs, angiotensin II receptor blockers, and ACE inhibitors on home BP variabilities, but they also reported that amlodipine reduced systolic BP variability in the morning compared with other CCBs [25]. Our findings suggest that cilnidipine has a similar capacity as amlodipine in reducing home BP variabilities, but the underlying mechanisms in reducing BP variabilities may be different between the two drugs.
In most previous clinical studies and actual medical practice, a half dose of amlodipine has been considered to be equivalent to a full dose of cilnidipine in reducing BP [19]. Therefore, we used this relationship when switching the test drugs in the present study. However, we found that the home BP during cilnidipine treatment was modestly, but significantly, higher than the BP with amlodipine treatment. The BP lowering effect of CCBs is determined by their capacity to reduce vascular resistance. The capacity of CCBs to reduce vascular resistance is also proposed to play a primary role in their ability to reduce BP variability [9, 13]. We found that amlodipine reduced evening BP variability more effectively than cilnidipine in patients with lower arterial stiffness determined by the baPWV. We also found that higher arterial stiffness was associated with higher evening BP variability during amlodipine treatment, which is consistent with the current concept that high arterial stiffness is associated with BP variability in the elderly [26]. Altogether, it is conceivable that the capacity of amlodipine to reduce vascular resistance is somewhat superior to cilnidipine, and the small difference makes amlodipine more effective than cilnidipine in reducing evening BP variability preferentially in patients with low arterial stiffness.
Several clinical and basic studies support the unique capacity of cilnidipine to reduce sympathetic nerve activity by blocking N-type calcium channels [15, 27]. In this study, we found that the pulse rate was significantly smaller during cilnidipine treatment than during amlodipine treatment, implying the effect of N-type calcium channel blockade by cilnidipine. The N-type calcium channel is expressed in nerve terminals, and it is involved in catecholamine secretion [15, 27]. Therefore, it is conceivable that patients with high catecholamine secretion receive a higher benefit from cilnidipine than those with low catecholamine secretion. Consistent with this notion, we found that high urinary catecholamine excretion is associated with the effectiveness of cilnidipine in reducing morning BP variability, compared to amlodipine.
Of note, our results suggest that measuring the time of the home BP influences the association between arterial stiffness and BP variability. We found a significant correlation between arterial stiffness and BP variability in the evening, which was not the case in the morning, suggesting that arterial stiffness is a potent determinant of evening BP variability. We also observed the preferential effect of cilnidipine in patients with high catecholamine excretion on BP variability in the morning, which was not the case in the evening. Interestingly, it was recently reported that evening BP variability predicted cardiovascular disease, while morning BP variability did not [25]. They showed that variability, independent of the mean and average variability, in the evening BP before sleep significantly predicted cardiovascular events independent of the BP level, while these parameters in the morning did not have an independent predictable ability for cardiovascular outcomes. It is also well-known that arterial stiffness predicts cardiovascular events in hypertensive elderly, which is independent of the BP level [28]. Taken together, it is conceivable that BP variability in the evening is a better prognostic factor than that in the morning because arterial stiffness is more purely reflected in BP variability in the evening than in the morning.
There are several limitations in the study. First, while the number of participants for evaluating the primary outcome was sufficient as described in the Method section, the number was insufficient to provide full reliability for some analyses. For example, the lack of a correlation between two variables in some of the figures needs to be validated in a larger sample size. Second, we found a difference in the BP level between amlodipine and cilnidipine treatments, suggesting that these drugs do not equivalently block L-type channels. Therefore, our results failed to draw a conclusion on whether blocking N-type calcium channels has an additional effect on home BP variability. Third, the subjects in the study are relatively healthy and have a low frequency of diabetes and chronic kidney disease. Further investigation will be required to compare the BP variability between amlodipine and cilnidipine in high-risk hypertensive patients. Fourth, the subjects were 65 years of age or older in this study, and did not include non-elderly patients. As non-elderly hypertensive patients tend to have low arterial stiffness and high sympathetic nerve activity compared to elderly patients, different findings could be withdrawn from the non-elderly patients.
In conclusion, we did not observe any difference in home BP variabilities between amlodipine and cilnidipine treatments in elderly hypertensive patients. Analysis based on the difference in the BP variability between the two drugs in individual patients provided results supporting the following hypothesis: High sympathetic nerve activity with increased catecholamine secretion is associated with the effectiveness of cilnidipine in reducing morning BP variability, and low arterial stiffness is associated with the effectiveness of amlodipine in reducing evening BP variability.
References
Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, Ferrario M, Mancia G. Blood pressure variability and organ damage in a general population–results from the PAMELA study. Hypertension. 2002;39:710–4.
Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045–50.
Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes I. Prognostic value of reading-to-reading blood pressure variability over 24h in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–57.
Niiranen TJ, Hanninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55:1346–51.
Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905.
Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, Pantazis N, Baibas NM. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158–64.
Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension. 2012;59:212–8.
Matsui Y, O’Rourke MF, Hoshide S, Ishikawa J, Shimada K, Kario K. Combined effect of angiotensin II receptor blocker and either a calcium channel blocker or diuretic on day-by-day variability of home blood pressure: the Japan Combined Treatment With Olmesartan and a Calcium-Channel Blocker Versus Olmesartan and Diuretics Randomized Efficacy Study. Hypertension. 2012;59:1132–8.
Ushigome E, Matsumoto S, Oyabu C, Ushigome H, Yokota I, Hasegawa G, Nakamura N, Tanaka M, Yamazaki M, Fukui M. Olmesartan with azelnidipine versus with trichlormethiazide on home blood pressure variability in patients with type II diabetes mellitus. J Am Soc Hypertens. 2017;11:140–147.
Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–48.
Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS. Effects of β blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–80.
Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–15.
Rakugi H, Ogihara T, Saruta T, Kawai T, Saito I, Teramukai S, Shimada K, Katayama S, Higaki J, Odawara M, Tanahashi N, Kimura G. Preferable effects of olmesartan/calcium channel blocker to olmesartan/diuretic on blood pressure variability in very elderly hypertension: COLM study subanalysis. J Hypertens. 2015;33:2165–72.
Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ, Tsien RW. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988;239:57–61.
Sakata K, Shirotani M, Yoshida H, Nawada R, Obayashi K, Togi K, Miho N. Effects of amlodipine and cilnidipine on cardiac sympathetic nervous system and neurohormonal status in essential hypertension. Hypertension. 1999;33:1447–52.
Konoshita T, Makino Y, Kimura T, Fujii M, Wakahara S, Arakawa K, Inoki I, Nakamura H, Miyamori I. A new-generation N/L-type calcium channel blocker leads to less activation of the renin-angiotensin system compared with conventional L type calcium channel blocker. J Hypertens. 2010;28:2156–60.
Kishi T, Hirooka Y, Konno S, Sunagawa K. Cilnidipine inhibits the sympathetic nerve activity and improves baroreflex sensitivity in patients with hypertension. Clin Exp Hypertens. 2009;31:241–9.
Morimoto S, Yano Y, Maki K, Iwasaka T. Renal and vascular protective effects of cilnidipine in patients with essential hypertension. J Hypertens. 2007;25:2178–83.
Fujita T, Ando K, Nishimura H, Ideura T, Yasuda G, Isshiki M, Takahashi K. Cilnidipine versus amlodipine randomised trial for evaluation in renal desease study I. Antiproteinuric effect of the calcium channel blocker cilnidipine added to renin-angiotensin inhibition in hypertensive patients with chronic renal disease. Kidney Int. 2007;72:1543–9.
Takahashi H, Yoshika M, Yokoi T. Validation of two automatic devices: Omron HEM-7252G-HP and Omron HEM-7251G for self-measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20:286–90.
Komai N, Ohishi M, Morishita R, Moriguchi A, Kaibe M, Matsumoto K, Rakugi H, Higaki J, Ogihara T. Serum hepatocyte growth factor concentration is correlated with the forearm vasodilator response in hypertensive patients. Am J Hypertens. 2002;15:499–506.
Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol. 2003;91:1519–22. a1519
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Yano Y, Rakugi H, Bakris GL, Lloyd-Jones DM, Oparil S, Saruta T, Shimada K, Matsuoka H, Imai Y, Ogihara T. On-treatment blood pressure and cardiovascular outcomes in older adults with isolated systolic hypertension. Hypertension. 2017;69:220–7.
Asayama K, Ohkubo T, Hanazawa T, Watabe D, Hosaka M, Satoh M, Yasui D, Staessen JA, Imai Y. Does antihypertensive drug class affect day-to-day variability of self-measured home blood pressure? The HOMED-BP Study. J Am Heart Assoc. 2016;5:e002995.
Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–72.
Molderings GJ, Likungu J, Gothert M. N-type calcium channels control sympathetic neurotransmission in human heart atrium. Circulation. 2000;101:403–7.
Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113:657–63.
Acknowledgements
We are very grateful to Yuka Nakao and Hikari Kitamura for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nozato, S., Yamamoto, K., Nozato, Y. et al. Comparison between L-type and N/L-type calcium channel blockers in the regulation of home blood-pressure variability in elderly hypertensive patients. Hypertens Res 41, 290–298 (2018). https://doi.org/10.1038/s41440-018-0018-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0018-4
This article is cited by
-
Novel Indices of Home Blood Pressure Variability and Hypertension-Mediated Organ Damage in Treated Hypertensive Patients
High Blood Pressure & Cardiovascular Prevention (2021)