Abstract
Cardiovascular autonomic nervous system function can be assessed using an orthostatic challenge to induce arterial wave reflection. While arterial reflection is typically estimated using a central augmentation index, a superior estimation can be obtained using pulse wave separation analysis to estimate the aortic backward pressure wave (Pb). However, to be of value in a clinical or research setting, an assessment tool must be precise (reliable). Therefore, this study sought to determine the measurement precision of Pb responses to a modified tilt-table test. Twenty healthy adults (26.4 year (SD 5.2), 24.7 kg/m2 (SD 3.8), 55% female) were tested in a fasted state on three different mornings separated by a maximum of seven days. Pressure waveforms were recorded on the left arm, and aortic waveforms were generated using a generalized transfer function. Subsequently, a physiologic flow waveform was assumed to separate the aortic pressure wave into its forward and timing-independent backward (Pb) components. The criterion intra-class correlation coefficient of ≥0.75 was exceeded at baseline (0.79), following a 5-min tilt (0.75), and following a 5-min recovery from tilt (0.75). The standard error of measurement was 7%. These findings indicate that in a healthy cohort, the Pb response to an orthostatic challenge can be assessed with acceptable precision. The next step is to determine the sensitivity (validity) of this technique in identifying cardiovascular autonomic dysfunction in patient groups.
Similar content being viewed by others
Introduction
A number of cardiovascular disturbances, including hypertension and stroke, have been linked to autonomic nervous system (ANS) dysfunction [1,2,3]. The ANS function can be assessed using an orthostatic challenge, during which a normally functioning ANS attempts to prevent pooling of blood in the sub-diaphragmatic venous system by inducing vasoconstriction of the resistance and capacitance vessels [4, 5]. The vasoconstrictor response contributes to increased arterial wave reflection, which can be assessed using pulse wave analysis (PWA). Recently, our group published an article that reported that oscillometric PWA can be used to measure arterial wave reflection responses to an orthostatic challenge with acceptable precision (between-day reliability) [6]. In our previous study, arterial reflection was estimated using the augmentation index (AIx), an indicator of central pressure augmentation. Arguably, the emerging pulse waveform separation analysis method would provide a superior index of arterial wave reflection.
The AIx, which is calculated by dividing the central augmentation pressure by the corresponding pulse pressure, is affected by the reflected wave transit time [7, 8]. The transit time is affected by the reflected wave timing, amplitude, and ventricular function, which in turn are known to be influenced by a number of factors, including heart rate [7, 8]. Alternatively, by assuming a triangular or a physiologic flow waveform, the aortic pressure wave (P) can be separated into its forward (Pf) and timing-independent backward (Pb) components [9]. Given that the backward (reflected) wave transit time likely fluctuates during an orthostatic challenge, the Pb may provide a superior index of arterial wave reflection under these conditions. However, to be of value in a clinical or research setting, an assessment tool must be precise.
Recently, our group published an article that reported that Pb can be assessed with acceptable precision under normal resting conditions [10]. However, no previous study has assessed the precision of Pb responses to an orthostatic challenge. Therefore, the data from our previous orthostatic challenge study [6] was re-analyzed using pulse wave separation analysis. The purpose of the current study was to determine whether the Pb responses to an orthostatic challenge (modified tilt-table test) exceed the criterion for acceptable measurement precision (between-day reliability).
Methods
This observation study was conducted in accordance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [11].
Participants
To ascertain the upper limit of reliability, a relatively homogenous cohort of 20 young (19–35 year) and healthy participants was recruited. Participants were excluded if they smoked, reported any known cardio-metabolic disorders, or were taking medications known to affect cardiovascular function. Ethical approval was obtained from the Massey University Human Ethics Committee, and all participants provided written informed consent prior to participating in the study.
Experimental design
Prior to beginning the study, participants were familiarized with all experimental procedures. Subsequently, participants were tested on three different days in a dimly lit, climate-controlled room between the hours of 7 am and 10 am. All participants were fasted, having consumed only water, and refrained from caffeine and supplement intake that morning and from strenuous physical activity and alcohol for 24 h prior to experimentation. The maximum duration between the first and last study visit was 7 days (mean: 3.2 days SD (1.8)), and women were tested on consecutive days to limit the possible confounding influence of menstrual cycle hormones. Following a 10-min rest period in the supine posture, baseline PWA assessments were collected. Participants were then rapidly (~1 s) tilted to a 60-degree upright position using a modified tilt-table for 5 min. During the tilt period, PWA assessments were collected at 2 and 5 min (Tilt2, Tilt5). The participant was returned to the supine position for a 5-min recovery period, during which PWA assessments were collected at 2 and 5 min (Rec2, Rec5). Previous studies using modified and full tilt-table assessments have shown 5 min to be a sufficient period for eliciting stable hemodynamic responses [6, 12, 13].
Pulse wave analysis
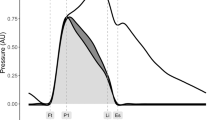
Oscillometric pressure waveforms were recorded on the left upper arm by a single observer using a SphygmoCor XCEL device (AtCor Medical, Sydney, Australia) following standard manufacturer guidelines [14]. Each measurement cycle lasted approximately 60 s, consisting of a brachial blood pressure recording and then a 10-s sub-systolic recording. A corresponding aortic pressure waveform (Fig. 1) was generated using a validated transfer function [15]. To enable direct comparison, the AIx data from the previous study were re-reported [6]. The AIx is defined as the augmentation pressure (AP), expressed as a percentage of central pulse pressure, where AP is defined as the maximum systolic pressure minus the pressure at the inflection point. The Pf and Pb wave pressures were determined by assuming a triangular flow wave [9, 16]. This method creates a triangular-shaped flow wave by matching the start, peak, and end of the flow wave to the timings of the foot, inflection point, and incisura of the aortic pressure wave (Fig. 1). Thus, the forward and backward components of the pressure wave can be constructed using the following equations:
Aortic pulse wave analysis. Using the generated aortic pressure waveform (top panel), the augmentation index (AIx) is calculated by expressing augmentation pressure (AP) as a percentage of the central pulse pressure (cPP). The AP is the additional pressure added to the forward wave by the reflected wave and is defined as the maximum central systolic pressure minus the pressure at the inflection point. Using a physiologic flow waveform (middle panel), the aortic wave can be separated (bottom panel) into its forward (Pf) and backward (Pb) waves, and reflection magnitude (RM) can be computed (Pb/Pf)
where P is the synthesized aortic pressure wave, Q is the approximated pseudo-flow wave, Zc is the characteristic impedance, Pf is the forward pressure component, and Pb is the backward pressure component. The RM was calculated as Pb/Pf. Because calculation of Pf and Pb involves the product of flow (Q) and characteristic impedance (Zc), which itself has flow in the denominator, calibration of the flow waveform is not needed.
Sample size
Sample size calculations were based on the primary outcome from the original study and the central systolic blood pressure and assumed a standard error of measurement of 2.5 mmHg derived from a previous reliability study using healthy subjects [17]. Using magnitude-based inferences [18] to estimate the sample size required to detect the smallest benefit (or detriment) in a crossover study, with the maximum chances of a type 1 and 2 error set at 5% (i.e., very unlikely), approximately 17 participants are required to detect a 2-mmHg change.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences version 22 (SPSS, Inc., Chicago, Illinois). All data are reported as the means and standard deviations (SD) unless specified otherwise. Statistical significance was defined as P < 0.05 (two-tailed). Reproducibility of parameters was assessed by calculating the intra-class correlation coefficient (ICC), standard error of measurement (SEM), and smallest detectable change (SDC). The ICC was calculated according to the formula SDb2/[SDb2 + SDw2], where SDb2 and SDw2 are the between-subject and within-subject variances. In general, ICC values above 0.75 are considered to indicate excellent reproducibility [19]. The SDC is defined as the critical difference in a parameter that must be exceeded between two sequential results for a statistically significant change to occur in an individual [20]. Absolute SDC was calculated using the formula 1.96 × SEM x √2, where 1.96 corresponds to 95% confidence interval, and SEM was calculated using the equation SDb x √(1-ICC) [20].
The effects of the orthostatic challenge on central hemodynamic parameters were assessed using analysis of variance (ANOVA) for repeated measurements with one within-subject factor (time: base, Tilt2, Tilt5, Rec2, Rec5). The participants were also sub-grouped according to whether Pb dropped (negative responder) or did not drop (positive responder) during the modified tilt-table. The group by time interactions were tested by adding a between-subjects factor (group) to the above models. A priori between-group Tilt5 responses were compared using independent samples t-tests. Effect sizes for ANOVA are reported using partial eta-squared (η2p), where 0.01, 0.06, and 0.14 represent small, medium, and large effects, respectively [21]. Effect sizes for the t tests are reported using Cohen’s d, where <0.20 is considered to be a small effect, >0.20 to <0.50 a moderate effect, and >0.60 a large effect.
Results
Twenty individuals were recruited, and complete data were collected for each stage of testing. Demographic data are shown in Table 1. There were no significant differences between negative and positive responders.
Reliability
Between-day reliability values are presented in Table 2. At baseline, all of the ICC values for all variables exceeded the criterion (0.75). During the modified tilt-table assessment (Tilt5), all variables except Pf exceeded the criterion ICC, and during recovery, only Pb and AIx exceeded the criterion.
Modified tilt-table responses
The Pb and Pf responses to the modified tilt-table are shown in Fig. 2, and the RM and AIx responses are shown in Fig. 3. There was a significant and large main effect for AIx (η2p = 0.23, Fig. 3a), with an absolute change of—5.3% (CI: −2.7, −7.9%). However, there were non-significant decreases in Pf and Pb (Fig. 2a) and in RM (Fig. 3a). Closer inspection of the individual data revealed that while AIx consistently decreased across participants, the Pb either decreased (negative responder) or increased (positive responder). When the groups were separated by responder status, there was a non-significant interaction for AIx (Fig. 3b), but medium effect interactions for Pf (Fig. 2b) and RM (Fig. 3c) and a large effect interaction for Pb (Fig. 2c) were observed.
Table 3 compares the modified tilt-table responses by negative versus positive responder. At baseline, there were no significant differences by group, and in response to the tilt-table, there were non-significant differences in peripheral hemodynamic variables. However, in response to the tilt-table, both groups exhibited drops in AIx (Interaction: p = 0.168, Fig. 3b), but there were medium effect opposing changes in Pf (P = 0.001, η2p = 0.28, Fig. 2b) and RM (p = 0.001, η2p = 0.13, Fig. 3c) and a large effect opposing change in Pb (P = 0.001, η2p = 0.13, Fig. 2c).
Discussion
This study demonstrates that the Pb responses to an orthostatic challenge (modified tilt-table) are acceptably precise (reliable) and that the level of precision at the group level is similar to that for reported AIx [6]. However, while the orthostatic challenge induced a large, significant change in AIx at the group level, there was a medium, non-significant effect for Pb. Subsequent analysis revealed that 65% of the group responded negatively (had a drop in Pb) to the challenge, while 35% of the group responded positively. We contend that the Pb responses are more physiologically plausible than the AIx responses and that the difference between the two parameters may be explained by technical considerations inherent to the calculation of AIx.
Previous tilt-table testing studies have shown total peripheral resistance to increase [22, 23] and stroke volume to decrease during the test [22, 23]. Increased total peripheral resistance is induced by the ANS, which increases the tone of the resistance and capacitance vessels in an attempt to prevent pooling of blood in the sub-diaphragmatic venous system [4, 5]. If venous pooling does occur, the subsequent drop in diastolic filling would result in diminished stroke volume, and the heart rate would increase to compensate. These physiological changes are in line with the results from the current study. The sub-group that experienced a drop in Pb (negative responders) during the orthostatic challenge (−1.7 vs. 0.7 mmHg, d = 2.6), likely as a result of venous pooling, also experienced a decrease in Pf (−1.7 vs. 0.8 mmHg, d = 1.40), arguably as a result of diminished stroke volume, and had the greatest compensatory increase in heart rate (4.5 vs. 1.7 bpm, d = 1.49). The small difference in Pb response observed between negative and positive responders likely reflects the normal inter-individual variability in sympathetic activation and the vascular responsiveness that may exist in healthy, young individuals [24]. Owing to technical considerations, the standard AIx parameter may have not detected these subtle changes between groups.
Two potential sources of error may have limited the estimation of arterial wave reflections using AIx: (i) the generalized transfer function used to generate the aortic pressure waveform and (ii) the reflected wave transit time. (i) The generalized transfer function may less faithfully reproduce the high-frequency components required for AIx computation than it does the low-frequency pressure harmonics required for Pb and Pf computation [25]. (ii) The AIx is affected by the reflected wave transit time, which is influenced by the reflected wave timing, amplitude, and ventricular function, and which are known to be influenced by a number of factors, including heart rate [7, 8, 26]. For the current study, heart rate, which increased by 4.3 (SD: 2.3) bpm and 1.7 (SD: 1.3) bpm for the negative and positive responders, respectively, may have acted as an additional source of variability, particularly for the negative responders. Thus, the decreased AIx during the orthostatic challenge may have not fully resulted from decreased wave reflection.
Implications and future direction
For the Pb Tilt5 response, the SDC, which is defined as the critical difference that must be exceeded for a significant change to occur in an individual [20], was 19.3% (2.3 mmHg). The group-mean decrease in Pb for the negative responders was 13% (1.7 mmHg). Therefore, while the SDC relative to the expected change was superior to that calculated for AIx (∆Tilt5: 6.7% vs SDC: 207%), Pb may not be sensitive enough to detect changes within an individual, at least for young, healthy individuals. However, unlike for AIx, the directionality of the Pb response may be physiologically plausible and of clinical utility. To confirm the physiological plausibility of Pb, future studies are required that simultaneously and continuously measure supporting hemodynamic variables, including stroke volume, heart rate, total peripheral resistance, and indicators of central sympathetic nervous system activity and peripheral sympathetic outflow.
For the original analysis, the intent was not to determine the directionality of the response; to ascertain the upper limit of reliability, we opted to recruit a homogenous cohort of young, healthy participants. To confirm the clinical plausibility of Pb responses to an orthostatic challenge, further study is required to determine the importance of age and cardiovascular health status. Though it must be recognized that there may not be a linear relationship between age, cardiovascular health status, cardiovascular ANS function, and risk of cardiovascular events. For example, with aging, while elevated sympatric outflow has been shown to occur, there is a decline in the capacity of sympathetic outflow to affect peripheral vascular responses [27]. Conversely, prolonged bed rest, as a model of extreme physical inactivity, leads to cardiovascular deconditioning and blunted baroreflex-mediated sympatho-excitation [28]. However, individuals with chronic heart failure experience excessive and sustained sympatho-excitation [29]. Further study is warranted to determine the clinical importance of these conflicting findings and to validate whether Pb can be used to determine the capacity of sympathetic outflow to direct peripheral vascular responses.
Of additional importance to clinical plausibility is the potential influence of sex. For the current study, while the physical characteristics for negative versus positive responders were similar, the positive responders included a high proportion of females (70 vs. 40%). While a full mechanistic explanation is beyond the scope of the current study, sex differences in cardio-vagal control have been reported [30, 31], including attenuated baroreflex-mediated increases in sympathetic activity [32]. The positive responders in the current study had a greater proportion of females, suggesting that an attenuated baroreflex-mediated increase in sympathetic activity was unlikely; however, this study was not powered to detect sex differences, and further investigation is warranted.
Conclusions
Cardiovascular ANS function can be assessed using an orthostatic challenge to induce arterial wave reflection. While arterial reflection is typically estimated using AIx, a superior estimation can arguably be provided by the emerging Pb. However, to be of value in a clinical or research setting, an assessment tool must be precise. The current study found that in a healthy cohort, (i) the precision (reliability) of the Pb response to an orthostatic challenge is acceptable and (ii) while future research is required to confirm physiological plausibility, the direction of the Pb response may be of greater clinical importance than the magnitude. The suggested next step is to determine whether the magnitude or the direction of the Pb response is most sensitive for identifying patients with cardiovascular autonomic dysfunction.
References
Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–66.
Korpelainen JT, Sotaniemi KA, Myllyla VV. Autonomic nervous system disorders in stroke. Clin Auton Res. 1999;9:325–33.
Tabara Y, Igase M, Miki T, Ohyagi Y, Matsuda F, Kohara K, Group JSs. Orthostatic hypertension as a predisposing factor for masked hypertension: the J-SHIPP study. Hypertens Res. 2016;39:664–9.
Freeman R. Assessment of cardiovascular autonomic function. Clin Neurophysiol. 2006;117:716–30.
Smit AA, Halliwill JR, Low PA, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999;519(Pt 1):1–10.
Stoner L, Bonner C, Credeur D, Lambrick D, Faulkner J, Wadsworth D, Williams MA. Reliability of oscillometric central hemodynamic responses to an orthostatic challenge. Atherosclerosis. 2015;241:761–5.
Mitchell GF. Triangulating the peaks of arterial pressure. Hypertension. 2006;48:543–5.
Stoner L, Faulkner J, Lowe A, M Lambrick D, M Young J, Love R, S Rowlands D. Should the augmentation index be normalized to heart rate? J Atheroscler Thromb. 2014;21:11–16.
Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension. 2006;48:595–601.
Stoner L, Credeur D, Fryer S, Faulkner J, Lambrick D, Gibbs BB. Reliability of pulse waveform separation analysis: effects of posture and fasting. J Hypertens. 2017;35:501–5.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7.
Montano A, Brown F, Credeur DP, Williams MA, Stoner L. Validity and relibaility of telemetry-derived heart rate variability responses to an orthostatic challenge. Eur J Clin Invest. 2017;37:421–7.
Castellano V, Olive JL, Stoner L, Black C, McCully KK. Blood flow response to a postural challenge in older men and women. Dyn Med. 2004;3:1.
Stoner L, Lambrick DM, Faulkner J, Young J. Guidelines for the use of pulse wave analysis in adults and children. J Atheroscler Thromb. 2013;20:404–6.
Butlin M, Qasem A, Avolio AP. Estimation of central aortic pressure waveform features derived from the brachial cuff volume displacement waveform. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:2591–4.
Qasem A, Avolio A. Determination of aortic pulse wave velocity from waveform decomposition of the central aortic pressure pulse. Hypertension. 2008;51:188–95.
Filipovsky J, Svobodova V, Pecen L. Reproducibility of radial pulse wave analysis in healthy subjects. J Hypertens. 2000;18:1033–40.
Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41:3–13.
Fleiss JL. The design and analysis of clinical experiments. New York: Wiley; 1986.
Fraser CG. Biological variation: from principles to practice. Washington, DC: AACC Press; 2001.
Cohen J. Statistical power analysis for the behavioral sciences. New York; London: Academic Press; 1969.
Tahvanainen A, Leskinen M, Koskela J, Ilveskoski E, Nordhausen K, Oja H, Kahonen M, Koobi T, Mustonen J, Porsti I. Ageing and cardiovascular responses to head-up tilt in healthy subjects. Atherosclerosis. 2009;207:445–51.
Tahvanainen A, Koskela J, Tikkakoski A, Lahtela J, Leskinen M, Kahonen M, Nieminen T, Koobi T, Mustonen J, Porsti I. Analysis of cardiovascular responses to passive head-up tilt using continuous pulse wave analysis and impedance cardiography. Scand J Clin Lab Invest. 2009;69:128–37.
Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–7.
Segers P, Carlier S, Pasquet A, Rabben SI, Hellevik LR, Remme E, De Backer T, De Sutter J, Thomas JD, Verdonck P. Individualizing the aorto-radial pressure transfer function: feasibility of a model-based approach. Am J Physiol Heart Circ Physiol. 2000;279:H542–549.
Hickson SS, Nichols WW, Yasmin, McDonnell BJ, Cockcroft JR, Wilkinson IB, McEniery CM. Influence of the central-to-peripheral arterial stiffness gradient on the timing and amplitude of wave reflections. Hypertens Res. 2016;39:723–9.
Taylor JA, Tan CO. BP regulation VI: elevated sympathetic outflow with human aging: hypertensive or homeostatic? Eur J Appl Physiol. 2014;114:511–9.
Hasser EM, Moffitt JA. Regulation of sympathetic nervous system function after cardiovascular deconditioning. Ann N Y Acad Sci. 2001;940:454–68.
Antoine S, Vaidya G, Imam H, Villarreal D. Pathophysiologic Mechanisms in Heart Failure: Role of the Sympathetic Nervous System. Am J Med Sci. 2017;353:27–30.
Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–8.
Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ. Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol. 2011;301:H2454–2465.
Foley CM, Mueller PJ, Hasser EM, Heesch CM. Hindlimb unloading and female gender attenuate baroreflex-mediated sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1440–1447.
Acknowledgements
We would like to acknowledge the assistance of Dr. Ahmed Qasem of AtCor, who kindly re-processed the original data set to calculate the wave separation variables.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Stoner, L., Stone, K., Hanson, E.D. et al. Reliability of pulse waveform separation analysis responses to an orthostatic challenge. Hypertens Res 41, 176–182 (2018). https://doi.org/10.1038/s41440-017-0005-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-017-0005-1
This article is cited by
-
Central and peripheral arterial stiffness responses to uninterrupted prolonged sitting combined with a high-fat meal: a randomized controlled crossover trial
Hypertension Research (2021)
-
The impact of upper-limb position on estimated central blood pressure waveforms
Journal of Human Hypertension (2019)