Abstract
Our previous report indicated that vascular injury enhances vascular remodeling in fetal growth restriction (FGR) mice. The angiotensin II type 2 receptor (AT2R) is relatively highly expressed in fetal mice. Therefore, we investigated the roles of AT2R in FGR-induced cardiovascular disease using AT2R knockout (AT2KO) mice. Dams (wild-type and AT2KO mice) were fed an isocaloric diet containing 20% protein (NP) or 8% protein (LP) until delivery. Arterial blood pressure, body weight, and histological changes in organs were investigated in offspring. The birth weight of offspring from dams fed an LP diet (LPO) was significantly lower than that of offspring from dams fed an NP diet. The heart/body and kidney/body weight ratios in AT2KO-LPO at 12 weeks of age were significantly higher than those in the other groups. Greater thickness of the left ventricular wall, larger cardiomyocyte size and enhancement of perivascular fibrosis were observed in AT2KO-LPO. Interestingly, mRNA expression of collagen I and inflammatory cytokines was markedly higher in the AT2KO-LPO heart at 6 weeks of age but not at 12 weeks of age. AT2R signaling may be involved in cardiovascular disorders of adult offspring with FGR. Regulation of AT2R could contribute to preventing future cardiovascular disease in FGR offspring.
Similar content being viewed by others
Introduction
Low birth weight, which is defined as birth weight under 2500 g, affects approximately 15% of all newborns world-wide [1]. Fetal growth restriction (FGR) is one of the main factors in low birth weight. FGR is caused by conditions such as maternal malnutrition, pregnancy-induced hypertension (preeclampsia), smoking, alcohol abuse, and drugs. FGR is a risk factor for high mortality and medical problems in early life [2]. Moreover, FGR is also a risk factor for disease in adulthood. A number of epidemiological and animal studies suggest that FGR is associated with an increased risk of cardiovascular disease and metabolic disorders in adulthood [3,4,5,6]. The concept that programming in early life affects adult health is known as “developmental origins of health and disease”.
Barker et al. reported the association between mortality from coronary heart disease and birth weight. Mortality is higher in FGR than in non-FGR adults [3]. Skilton et al. reported that aortic intima-media thickness in the neonate is greater in FGR [7]. Martyn et al. reported that the risk of carotid stenosis at 70 years old is higher in FGR [8]. In basic studies, Vonnahme et al. reported that fetal sheep with maternal undernutrition-induced FGR showed ventricular hypertrophy and increased liver weight [9]. Recently, using a cuff placement model, we also reported that maternal low-protein (LP) diet-induced FGR promotes vascular remodeling, [10]. Therefore, FGR is associated with cardiovascular disease in adulthood, but the mechanism is still unclear.
The renin-angiotensin system (RAS) plays important roles in physiological control of the cardiovascular and renal systems [11]. The two phenotypes of the angiotensin II receptor are known as angiotensin II type 1 receptor (AT1R) and type 2 receptor (AT2R). The functions of AT1R include vasoconstriction, elevation of blood pressure, thickening of the vessel wall, arteriosclerotic action, and cardiac hypertrophy. On the other hand, AT2R has a protective role against cardiovascular disease. AT2R is more highly expressed in fetal tissues than in adult tissues and is considered to play a role in organ development of the fetus [12]. Wang et al. reported that the kidneys showed more apoptotic cells in FGR than in non-FGR, and both renin and angiotensinogen decreased in the FGR group 13]. Moreover, our previous report demonstrated that mice overexpressing AT1R-associated protein (ATRAP), which promotes AT1R internalization and attenuates angiotensin II-mediated function, did not show an increase in blood pressure in an LP diet-induced FGR model, while blood pressure elevation was observed in wild-type (WT) LP-diet-induced FGR mice [14]. These data suggest an interaction between the RAS and FGR-induced cardiovascular disease. Although there are several reports focusing on the RAS in dams, such as studies of pregnancy-associated hypertension and FGR, we did not find studies that investigated the effect of the RAS on FGR-offspring using genetically modified mice. Here, focusing on the role of AT2R in FGR offspring, we investigated the difference in cardiovascular organs between WT and AT2R knockout mice (AT2KO) mice in an LP-diet-induced FGR model.
Methods
This study was performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. All experimental protocols were approved by the Animal Studies Committee of Ehime University.
Animals and treatment
Male C57BL/6J strain (WT and AT2KO) mice were used in this study. The animals were housed in a room in which lighting was controlled (12 h on, 12 h off) and room temperature was kept at 25 ℃. FGR was induced by maternal protein restriction. Dams were fed an isocaloric diet containing 20% protein (normal protein; NP, Oriental Yeast, Tokyo, Japan) or 8% protein (LP, Oriental Yeast) from 10 weeks of age until delivery. On the day of delivery, all dams were returned to the NP diet. At 2, 6, and 12 weeks of age, we measured the offspring body and organ weights, such as the heart, liver, kidney and brain. At 12 weeks of age, offspring heart rate and blood pressure were measured using the tail-cuff method (MK2000ST; Muromachi Kikai Co., Tokyo, Japan).
Morphometric analysis
Formalin-fixed, paraffin-embedded sections of hearts were prepared at 6 and 12 weeks of age. The middle segment of the heart was cut into cross sections. The sections were stained with hematoxylin-eosin for measurement of left ventricular wall thickness and cross-sectional area of cardiomyocytes and by van Gieson’s method for measurement of interstitial and perivascular fibrosis. Perivascular fibrosis was expressed as the ratio of the area of perivascular fibers to the area of the coronary artery. Measurement of areas was performed using image analysis software (Densitograph; ATTO Corporation, Tokyo, Japan).
Real-Time RT-PCR
Total RNA was extracted from hearts using Sepasol RNA I Super G (Nacalai tesque Inc., Kyoto, Japan). Real-time quantitative reverse-transcription polymerase chain reaction (PCR) was performed with a SYBR Premix Ex Taq kit (Takara Bio Inc., Shiga, Japan). PCR primers were as follows: AT1 receptor, 5′-AGTCGCACTCAAGCCTGTCT-3′ (forward) and 5′-ACTGGTCCTTTGGTCGTGAG-3′ (reverse); AT2 receptor, 5′-CCTGCATGAGTGTCGATAGGT-3′ (forward) and 5′-CCAGCAGACCACTGAGCATA-3′ (reverse); interleukin (IL)-1β, 5′-TCCAGGATGAGGACATGAGCAC-3′ (forward) and 5′-GAACGTCACACACCAGCAGGTTA-3′ (reverse); IL-6, 5′-CCACTTCACAAGTCGGAGGCTTA-3′ (forward) and 5′-GCAAGTGCATCATCGTTGTTCATAC-3′ (reverse); monocyte chemotactic protein-1, 5′-TTAACGCCCCACTCACCTGCTG-3′ (forward) and 5′-GCTTCTTTGGGACACCTGCTGC-3′ (reverse); tumor necrosis factor (TNF)-α, 5′-ATGTAGGCCATGAGGTCCAC-3′ (forward) and 5′-TGCGACTTCAACAGCAACTC-3′ (reverse); collagen I, 5′-ATCTCCTGGTGCTGAGGAC-3′ (forward) and 5′-ACCTTGTTTGCCAGGTTCAC-3′ (reverse); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-ATGTAGGCCATGAGGTCCAC-3′ (forward) and 5′-TGCGACTTCAACAGCAACTC-3′ (reverse).
Cuff-induced vascular injury model
When offspring were 10 weeks of age, inflammatory vascular injury was induced by polyethylene cuff placement around the femoral artery under anesthesia with intraperitoneal injection of 60 mg/kg pentobarbital sodium in saline, and morphometric analysis to measure the neointimal area was performed as described previously [10].
Statistical analysis
All values are expressed as the mean ± standard deviation in the text and figures. The data were analyzed by analysis of variance. If a statistically significant effect was found, post hoc analysis was performed with a Tukey-Kramer test to detect the difference. Values of p less than .05 were considered statistically significant.
Results
Effect of LP diet in dams on physical parameters of offspring
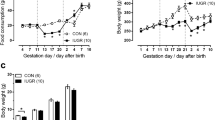
The average birth weight was significantly lower in LP offspring (LPO) compared with normal-protein (NP) offspring (NPO) in both WT and AT2KO mice (Fig. 1a). The body weight of LPO caught up at 2 weeks of age. Thereafter, there was no significant difference in body weight among the groups (Fig. 1b). Heart rate and systolic blood pressure evaluated using the tail-cuff method at 12 weeks of age did not differ among the groups (Fig. 1c, d).
a Birth weight of male C57BL/6J mice (WT) and AT2R knockout mice (AT2KO) offspring from mothers fed a normal protein (NPO) and a low protein (LPO) diet. The values are the mean ± SD (n = 38 to 76 for each group). **p < 0.01 vs. NPO. b Body weight of male offspring at 0, 2, 4, 8, and 10 weeks of age. After 2 weeks of age, there is no significant difference in body weight. Heart rate (c) and systolic blood pressure (d) at 12 weeks of age. The values are the mean ± SD (n = 5 to 7 for each group)
Effect of low protein diet in dams on organ weights of offspring
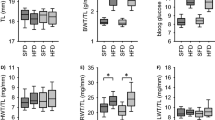
We examined the weights of organs, such as the heart, kidney, liver and brain, at 2, 6, and 12 weeks of age (Fig. 2). Organ weight to body weight ratios did not differ among the groups until 6 weeks of age; however, heart weight to body weight ratio (HW/BW ratio) and kidney weight to body weight ratio (KW/BW ratio) at 12 weeks of age were significantly higher in AT2KO-LPO than in the other groups (Fig. 2a, b). On the other hand, liver weight to body weight ratio did not differ among the groups (Fig. 2c). The brain weight to body weight ratio of the LPO groups at 2 weeks of age was significantly higher than that of the NPO groups (Fig. 2d), but actual brain weight did not differ among the groups (Fig. 2e).
Time course of organ-to-body weight ratio of kidney (a), heart (b), liver (c), and brain (d) and brain weight (e) at 2, 6, and 12 weeks of age. The values are the mean ± SD (n = 4 to 11 for each group). *p < 0.05 and **p < 0.01 vs. other groups at the same age. Light gray; WT-NPO, black; WT-LPO, white; AT2KO-NPO, dark gray; AT2KO-LPO
AT2KO-LP offspring exhibited cardiomegaly and perivascular fibrosis in the heart
Analysis of hematoxylin-eosin-stained cross-sections of the heart at 12 weeks of age indicated that AT2KO-LPO mice showed a significant increase in left ventricular wall area to body weight ratio compared with WT-NPO and AT2KO-NPO (Fig. 3a, b). Moreover, the cross-sectional area of cardiomyocytes was significantly greater in AT2KO-LPO mice than in the other groups (Fig. 3c, d).
Cardiac hypertrophy at 12 weeks of age. Representative photos of hematoxylin-eosin staining of cross-sections of the heart at a macro view (a) or at high magnification (c). Left ventricular area to body weight ratio (b) and cross-sectional area of cardiomyocytes (d). The values are the mean ± SD (n = 4–10 for each group). *p < 0.05 and **p < 0.01 vs. NPO (Color figure online)
On the other hand, the collagen fibers around coronary arteries in mice at 12 weeks of age were shown by van Gieson’s staining of sections of the heart (Fig. 4a). The fibrosis area to coronary artery area ratio in AT2KO-LPO was significantly higher than that in the other groups (Fig. 4b), while interstitial fibrosis was not different among the groups. To clarify the mechanism, we assessed the mRNA expression of collagen Ι in the heart at 6 weeks of age. Collagen I was significantly more highly expressed in AT2KO-LPO than in AT2KO-NPO, whereas there was no significant difference in expression in each mouse at 12 weeks of age (Fig. 4c).
Cardiac fibrosis at 12 weeks of age. a Representative photos of perivascular fibrosis of the heart with van Gieson’s staining. b Fibrosis area/coronary artery area ratio. c mRNA expression of collagen I. The values are the mean ± SD (n = 4 to 5 for each group). **p < 0.01 vs. NPO (Color figure online)
AT2KO-LP offspring showed increased expression of inflammatory cytokines in the heart
Next, we examined the mRNA expression of AT1R and AT2R at 6 and 12 weeks of age. At 12 weeks of age, there was no significant difference in the expression of angiotensin receptors in each group. The expression of AT1R at 6 weeks of age in the AT2KO group was significantly higher than in the WT group (Fig. 5a, b). Moreover, we analyzed the effect of FGR on mRNA levels of proinflammatory cytokines in the heart at 6 and 12 weeks of age using real-time quantitative RT-PCR (Fig. 5c–f). At both 6 and 12 weeks of age, the mRNA expression level of MCP-1 was significantly higher in AT2KO-LPO than in the other groups (Fig. 5d). At 12 weeks of age, the mRNA expression of IL-1β, IL-6, and TNF-α was not different among the groups; however, at 6 weeks of age, these expression levels were significantly higher in AT2KO-LPO than in the other groups (Fig. 5c, e, f).
AT2KO-LP offspring did not show an increase in neointimal formation in the cuff-induced vascular remodeling model
Finally, we investigated inflammation-induced vascular remodeling as described previously [10]. In a previous report, neointimal formation in the femoral artery was exaggerated in LPO compared with NPO in WT mice (Fig. 6). However, in AT2KO mice, LP-induced FGR did not affect neointimal formation, while AT2KO-LPO showed an increase in neointimal formation, as noted in our previous report (Fig. 6) [15].
Discussion
Here, we demonstrated that AT2R is involved in LP-diet-induced cardiac hypertrophy, with an increase in inflammatory cytokines, while there was no significant difference in birthweight and blood pressure between mice with and without AT2R expression. The results of the present study show the role of AT2R in FGR-induced cardiovascular changes.
Utsunomiya et al. reported that expression of AT2R in the heart was restricted to the coronary blood vessels [16]. In our data, the ratio of perivascular fibrosis to coronary artery area was enhanced in AT2KO-LPO, but there was no difference in interstitial fibrosis. These data suggest that AT2R plays a protective role against fibrosis, especially in the coronary vessels. In the present study, AT2KO-NPO did not exhibit a significant change in cardiac development, while FGR induced such a change in AT2KO mice. Our previous report regarding angiotensin-converting enzyme 2 (ACE2)-deficient (ACE2KO) mice demonstrated that deletion of ACE2 also increases heart weight, coronary artery thickening and perivascular fibrosis [17]. This change was observed from 4 weeks of age. Although in this paper, a low-protein diet was not employed in ACE2-deficient mice, RAS components could play an important role in cardiac development, especially in development of coronary arteries. Very recently, Rabelo et al. reported that deletion of ACE2 induces vascular dysfunction through nitric oxide (NO) imbalance and oxidative stress [18]. Interestingly, Shukla et al. demonstrated that maternal nutrient restriction during pregnancy impairs an endothelium-derived hyperpolarizing factor-like pathway in sheep fetal coronary arteries [19]. AT2R signaling is well known to have vasodilator activity, dependent on the bradykinin–bradykinin B2 receptor-NO-cyclic guanosine monophosphate (cGMP) pathway [20]. Therefore, deletion of AT2R could prevent protection against LP-induced endothelial dysfunction through dysfunction of the bradykinin-bradykinin B2 receptor-NO-cGMP pathway. Our examination of oxidative stress-related effects, such as expression of NADPH subunits, did not show a significant change in AT2KO-LPO. If we had been able to analyze NADPH subunit expression in dissected arteries to separate the coronary arteries from cardiac tissue, some differences might have been observed.
On the other hand, AT2R-induced vasorelaxation, natriuresis, antigrowth, and anti-inflammatory effects occur exclusively in women [21]. Our previous report demonstrated that neointimal formation is decreased in female mice compared with that in male mice through an increase in AT2 receptor expression [22]. Therefore, female mice could show a greater difference in cardiovascular change in FGR offspring. However, no significant difference in hypertrophy was observed between female AT2KO-LPO and AT2KO-NPO (data not shown), indicating that no advantage of the cardio-protective effect of AT2R was observed in female mice. Alternatively, it is possible that deletion of AT2R in the dam affects uterine artery function and induces epigenetic changes in the offspring. Acute, progressive endothelial dysfunction is observed in women with preeclampsia 23]. Mice lacking endothelial NO synthase exhibit a preeclampsia/FGR-like phenotype [24]. Therefore, an LP-diet may exaggerate uterine artery dysfunction in AT2KO dams and result in epigenetic modification in offspring. Considering that AT2KO-LPO did not show a lower birthweight than WT-LPO, uterine artery function was not different between WT and AT2KO mice; however, further investigation is necessary to assess the detailed mechanism to determine whether FGR-induced cardiovascular change was induced by the lack of AT2R in dams, offspring, or both.
On the other hand, we observed an increase in kidney weight/body weight in AT2KO-LPO at 12 weeks of age. We previously reported that mice with overexpression of ATRAP, which reduces AT1R signaling, show a slight but not significant increase in kidney weight/body weight ratio at 24 weeks of age with or without an LP diet [14]. Thus, AT1 receptor signaling may not contribute to this result. Growth-stimulatory effects by angiotensin II through AT1R may be counterbalanced by AT2 receptor-mediated apoptosis and growth inhibition [25]. AT2KO mice exhibit abnormal ureteric bud budding, increased incidence of double ureters and vesicoureteral reflux with attenuated apoptosis of mesenchymal cells during fetal metanephrogenesis [26]. Ablation of cap mesenchyme generated by condensation of the mesenchymal cells on the ureteric bud in early fetal kidneys causes progressive renal hypoplasia [27]. Therefore, one possible mechanism for this result is that lack of AT2-receptor-induced apoptosis in mesenchymal cells may increase kidney weight; however, the detailed mechanism is unclear.
Neointimal formation was not significantly different between AT2KO-LPO and AT2KO-NPO, although we expected more exaggerated vascular remodeling in AT2KO-LPO than in AT2KO-NPO. This result indicates that vascular remodeling is more strongly influenced by deletion of AT2R itself than by environmental factors, because this model is induced by subacute stress-induced vascular injury involving the vasculature and inflammatory cells. In this model, the genetic factor in offspring is superior to the FGR-induced effect. Moreover, AT2KO mice are systemic gene-deletion mice; therefore, various factors, such as cardiomyocytes, vasculature, macrophages and immune cells, are involved in these phenomena. Mice with more specific gene modification should be employed in future research.
In the present study, of the absence of AT2R is involved in cardiovascular disorders of adult offspring with FGR. However, it is not well known that “AT2R stimulation” is one of the prevention approaches for FGR-induced future cardiovascular disease. A relative increase in AT2R signaling by treatment with an angiotensin converting enzyme inhibitor or AT1R blocker (ARB) is considered to be a prevention approach. However, administration of an RAS blockade agent is contraindicated due to their potential fetotoxicity, such as oligohydramnios, as reported by Shimada et al. [28]. Recently, compound 21, a direct AT2 receptor stimulator, has been demonstrated to contribute to protection against end-organ damage in animal studies [29]. Although the teratogenic effects are not well elucidated, future investigation of treatment with compound 21 may be necessary.
In conclusion, these results suggest that AT2R signaling may be involved in cardiac development in adult offspring with FGR. Therefore, although AT1R blockers cannot be used in the gestational period because of the teratogenic risk, regulation of AT2R could contribute to prevention of future cardiovascular disease in FGR offspring.
References
Organization UNCsFaWH. Low birth weight: country, regional and global estimates. UNICEF, WHO: New York, 2004.
McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–8.
Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–7.
Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94:3246–50.
Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:1344S–52S.
Remacle C, Bieswal F, Bol V, Reusens B. Developmental programming of adult obesity and cardiovascular disease in rodents by maternal nutrition imbalance. Am J Clin Nutr. 2011;94:1846S–52S.
Skilton MR, Evans N, Griffiths KA, Harmer JA, Celermajer DS. Aortic wall thickness in newborns with intrauterine growth restriction. Lancet. 2005;365:1484–6.
Martyn CN, Gale CR, Jespersen S, Sherriff SB. Impaired fetal growth and atherosclerosis of carotid and peripheral arteries. Lancet. 1998;352:173–8.
Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, Murdoch WJ, Nijland MJ, Skinner DC, Nathanielsz PW, Ford SP. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod. 2003;69:133–40.
Chisaka T, Mogi M, Nakaoka H, Kan-No H, Tsukuda K, Wang XL, Bai HY, Shan BS, Kukida M, Iwanami J, Higaki T, Ishii E, Horiuchi M. Low-protein diet-induced fetal growth restriction leads to exaggerated proliferative response to vascular injury in postnatal life. Am J Hypertens. 2016;29:54–62.
de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72.
Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991;88:921–33.
Wang YP, Chen X, Zhang ZK, Cui HY, Wang P, Wang Y. Increased renal apoptosis and reduced renin-angiotensin system in fetal growth restriction. J Renin Angiotensin Aldosterone Syst. 2016;17:1–7.
Tsukuda K, Mogi M, Iwanami J, Min LJ, Jing F, Ohshima K, Horiuchi M. Influence of angiotensin II type 1 receptor-associated protein on prenatal development and adult hypertension after maternal dietary protein restriction during pregnancy. J Am Soc Hypertens. 2012;6:324–30.
Akishita M, Horiuchi M, Yamada H, Zhang L, Shirakami G, Tamura K, Ouchi Y, Dzau VJ. Inflammation influences vascular remodeling through AT2 receptor expression and signaling. Physiol Genom. 2000;2:13–20.
Utsunomiya H, Nakamura M, Kakudo K, Inagami T, Tamura M. Angiotensin II AT2 receptor localization in cardiovascular tissues by its antibody developed in AT2 gene-deleted mice. Regul Pept. 2005;126:155–61.
Moritani T, Iwai M, Kanno H, Nakaoka H, Iwanami J, Higaki T, Ishii E, Horiuchi M. ACE2 deficiency induced perivascular fibrosis and cardiac hypertrophy during postnatal development in mice. J Am Soc Hypertens. 2013;7:259–66.
Rabelo LA, Todiras M, Nunes-Souza V, Qadri F, Szijarto IA, Gollasch M, Penninger JM, Bader M, Santos RA, Alenina N. Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS One. 2016;11:e0150255.
Shukla P, Ghatta S, Dubey N, Lemley CO, Johnson ML, Modgil A, Vonnahme K, Caton JS, Reynolds LP, Sun C, O’Rourke ST. Maternal nutrient restriction during pregnancy impairs an endothelium-derived hyperpolarizing factor-like pathway in sheep fetal coronary arteries. Am J Physiol Heart Circ Physiol. 2014;307:H134–142.
Batenburg WW, Tom B, Schuijt MP, Danser AH. Angiotensin II type 2 receptor-mediated vasodilation. Focus on bradykinin, no and endothelium-derived hyperpolarizing factor(s). Vasc Pharmacol. 2005;42:109–18.
Hilliard LM, Mirabito KM, Denton KM. Unmasking the potential of the angiotensin AT2 receptor as a therapeutic target in hypertension in men and women: what we know and what we still need to find out. Clin Exp Pharmacol Physiol. 2013;40:542–50.
Okumura M, Iwai M, Nakaoka H, Sone H, Kanno H, Senba I, Ito M, Horiuchi M. Possible involvement of AT2 receptor dysfunction in age-related gender difference in vascular remodeling. J Am Soc Hypertens. 2011;5:76–84.
Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;Suppl A:S38–42.
Kusinski LC, Stanley JL, Dilworth MR, Hirt CJ, Andersson IJ, Renshall LJ, Baker BC, Baker PN, Sibley CP, Wareing M, Glazier JD. Enos knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am J Physiol Regul Integr Comp Physiol. 2012;303:R86–93.
Wolf G. Angiotensin II and tubular development. Nephrol Dial Transplant. 2002;Suppl9:48–51.
Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA, 3rd, Hogan BM, Fogo A, Brock JW, 3rd, Inagami T, Ichikawa I, Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell. 1999;3:1–10.
Cebrian C, Asai N, D’Agati V, Costantini F. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep. 2014;7:127–37.
Shimada C, Akaishi R, Cho K, Morikawa M, Kaneshi Y, Yamda T, Minakami H. Outcomes of 83 fetuses exposed to angiotensin receptor blockers during the second or third trimesters: a literature review. Hypertens Res. 2015;38:308–13.
Danyel LA, Schmerler P, Paulis L, Unger T, Steckelings UM. Impact of AT2-receptor stimulation on vascular biology, kidney function, and blood pressure. Integr Blood Press Control. 2013;6:153–61.
Acknowledgements
This study was supported by JSPS KAKENHI [Grant Number 25293310 to MH, 25462220 to MM, 15K19974 to JI, and 26860567 to LJM], and research grants from pharmaceutical companies: Astellas Pharma Inc., Daiichi-Sankyo Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma KK and Takeda Pharmaceutical Co., Ltd. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yamauchi, T., Mogi, M., Kan-no, H. et al. Roles of angiotensin II type 2 receptor in mice with fetal growth restriction. Hypertens Res 41, 157–164 (2018). https://doi.org/10.1038/s41440-017-0004-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-017-0004-2
This article is cited by
-
Effect of estrogen on fetal programming in offspring from high-fat-fed mothers
Hypertension Research (2022)