Abstract
Purpose
Cardiac–valvular and vascular Ehlers–Danlos syndrome (EDS) have significant cardiovascular issues. The prevalence and significance of such abnormalities in classical (cEDS) or hypermobile EDS (hEDS) remain unclear. We report the prevalence of cardiac abnormalities in patients with cEDS and hEDS.
Methods
We identified 532 pediatric patients with potential EDS evaluated at our institution from January 2014 through April 2019 by retrospective chart review. Ninety-five patients (12 cEDS and 83 hEDS patients) met 2017 EDS diagnostic criteria and had an echocardiogram. One patient was excluded due to complex congenital heart disease, and two were excluded due to lack of images. We reviewed echocardiograms for all structural abnormalities.
Results
Of these 95 patients, 1 had mild aortic root dilation, and 1 had mild ascending aorta dilation in the setting of a bicuspid aortic valve. Eleven patients (11.6%) had a cardiac valve abnormality, all of which were trivial to mild. None of the patients required cardiac intervention.
Conclusion
Our results demonstrate that aortic dilation and valvular anomalies are uncommon in cEDS or hEDS patients. Given the lack of evidence, we do not recommend echocardiographic evaluation and surveillance in patients with cEDS and hEDS in the absence of clinical findings or positive family history.
Similar content being viewed by others
INTRODUCTION
Ehlers–Danlos syndromes (EDS) are a collection of multisystem, heritable connective tissue disorders characterized by the presence of joint hypermobility, skin hyperextensibility, and tissue fragility.1 In addition to these cardinal features, there are other clinical manifestations of EDS that, with the presence of associated genetic defects, provide for the differentiation of EDS into multiple types. In the 1970s, there were six identified types of EDS2 that remained in place until the Berlin nosology delineated 11 types of EDS.3 Subsequently, a revised classification scheme with a greater molecular basis was devised, returning to six identified subtypes.4 Most recently, a new, revised nosology has been developed that identifies 13 types of EDS and has become the diagnostic standard.1 The new nosology has greater stringency in the diagnostic criteria, thereby increasing specificity of diagnoses.
Cardiovascular findings began to be increasingly reported after Cabeen et al. reported mitral valve prolapse and conduction abnormalities in a patient with a then (1977) diagnosis of type III EDS.5 In 1980, Leier et al. reported on cardiac defects in 19 patients with types I (classical) and III (hypermobile) EDS, with mitral and/or tricuspid valve prolapse being the most common finding, followed by aortic dilation.6 Subsequently, studies have been discrepant as to the presence and/or degree of cardiovascular involvement.7,8,9,10 In light of the prior studies, (Table 1) the most recent diagnostic guidelines for hypermobile EDS include aortic root dilation and mitral valve prolapse as criteria in the diagnosis.1
The experience in our large, pediatric connective tissue disorder program led us to question the degree of cardiovascular involvement in patients with either classical EDS (cEDS) or hypermobile EDS (hEDS). Given that previous studies were performed prior to the development of the current nosology and diagnostic criteria, and identification of the cardiac–valvular form of EDS,1 we hypothesized those studies might have incorrectly classified some patients as having either cEDS or hEDS, thus falsely elevating the prevalence of cardiovascular involvement. In this study, utilizing strict adherence to current diagnostic criteria for cEDS and hEDS, we sought to determine the prevalence of structural cardiovascular abnormalities in cEDS or hEDS patients evaluated at our institution.
MATERIALS AND METHODS
Subjects
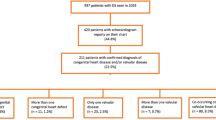
We performed a retrospective review of the electronic medical record from January 2014 through April 2019 for all patients at Lucile Packard Children’s Hospital Stanford who were clinically suspected to have either cEDS or hEDS (Fig. 1). Our inclusion criteria were: (1) patients met cEDS or hEDS diagnostic criteria based on the 2017 guidelines,1 and (2) patients had undergone an echocardiogram as part of their clinical evaluation. Two subjects were excluded based on a subsequent identification of a more severe syndrome (one with spondylodysplastic EDS, and one with an FLNA variant associated with periventricular nodular heterotopia, which is no longer considered a form of EDS based on the 2017 guidelines). An additional subject was excluded due to additional severe non-EDS phenotypes who was recommended to undergo exome analysis. One subject was excluded due to the presence of double outlet right ventricle, a form of complex congenital heart disease with a known association with aortic dilation. Two additional subjects were excluded due to lack of echocardiogram images available for review. The remaining potential subjects were excluded based on lack of evidence to satisfy the 2017 criteria for a diagnosis of cEDS or hEDS, including patients whose clinical features were more compatible with a diagnosis of hypermobility spectrum disorder.
Ethics statement
This study was reviewed and approved by the Stanford University Institutional Review Board.
Analysis of echocardiograms
The study echocardiograms were reviewed by two independent pediatric cardiologists with advanced training in echocardiography (E.S.S.T. and R.T.C.). In accordance with the guidelines of the American Society of Echocardiography, aortic dimensions were measured at the annulus, sinus of Valsalva (aortic root), sinotubular junction, and ascending aorta on parasternal long-axis images from inner edge to inner edge during systole.11 Absolute measurements of the aorta in combination with patient age, height, and weight were used to calculate Z-scores in the Boston Z-score database.12 We also assessed the echocardiograms for valvular abnormalities, including stenosis, regurgitation, redundancy, and prolapse. Interreader variability for the study echocardiographic measurements was <10%. Measurements were made on the initial echocardiogram for all subjects. For subjects with multiple serial echocardiograms, these were reviewed for any change in the Z-scores of aortic measurements or valvular dysfunction.
RESULTS
A total of 532 pediatric patients with a suspected diagnosis of cEDS or hEDS were evaluated at our institution during the 5-year study period. After excluding patients who did not meet 2017 EDS diagnostic criteria, had alternative diagnoses, had potentially confounding congenital heart disease, or did not have echocardiogram images available for review, the study cohort consisted of 95 subjects (73/95 female, 76.8%) with a confirmed diagnosis of either cEDS (12/95, 12.6%) or hEDS (83/95, 87.4%). The mean patient age at the time of the echocardiogram was 13.4 ± 4.4 years.
Aortic dimensions
All 95 subjects with cEDS or hEDS in our cohort had at least one echocardiogram for review. Follow-up echocardiograms were available in 29/95 (30.5%). Table 2 reports the aortic Z-scores in study subjects with cEDS or hEDS. As shown in Fig. 2, we observed a roughly normal distribution of aortic measurements, similar to that of the general population, with rare measurements exceeding a Z-score of 2. One subject had aortic root enlargement (Z-score = 2.36), which notably normalized on follow-up echocardiograms. One subject had mild ascending aorta dilation (Z-score = 2.52) in the setting of bicuspid aortic valve, a known association.13 Additionally, as demonstrated in Fig. 3, aortic dimension Z-scores showed no relationship to age.
Z-scores were calculated using the Boston Z-score methodology for the aortic valve annulus, aortic sinus of Valsalva (aortic root), sinotubular junction, and ascending aorta. All parameters show a roughly normal distribution around a mean (solid lines) slightly less than zero, indicating there is no significant difference between cEDS/hEDS patients and the general population. Of note, one of the two patients with an ascending aortic Z-score >2 had a bicuspid aortic valve, which is known to be associated with ascending aorta dilation.
In many connective tissue disorders with aortic involvement, the aortic enlargement is often apparent and more rapidly progressive during puberty. Here, we show that classical Ehlers–Danlos syndrome (cEDS) and hypermobile Ehlers–Danlos syndrome (hEDS) patients who have reached late adolescence do not have higher aortic Z-scores compared with younger patients.
Valvular assessment
Eleven patients (11.6%) had trivial-to-mild abnormalities of at least one cardiac valve (Table S1). Only one of the valvular abnormalities in the study cohort—the incidental finding of bicuspid aortic valve—would be expected to need ongoing cardiological follow-up.
DISCUSSION
Following stringent adherence to the 2017 diagnostic criteria for cEDS and hEDS, and relying on expert review of the echocardiographic images instead of chart review, we have shown that cardiovascular involvement in pediatric patients is rare, not of hemodynamic significance, and is generally not of a type that necessitates ongoing cardiovascular follow-up. Additionally, we found the majority of those patients with suspected cEDS and hEDS do not meet diagnostic criteria for EDS. Our results indicate that patients with confirmed or suspected cEDS and hEDS do not warrant cardiac evaluation unless there are clinical symptoms or a family history of aortopathy.
We did not find any clinically significant aortic abnormalities in our study cohort. While there were two patients who had evidence of mild aortic dilation, there are two important considerations to be made. The determination of aortic dilation is based on the statistical construct of Z-scores. In any population with a normal distribution of aortic diameters, we expect there to be 2.3% of that cohort whose aorta exceeds two standard deviations above the mean of zero. As such, in a study group of 95 subjects with a normal distribution, we would expect two subjects to have a Z-score above the “normal” maximum cutoff of 2, which is consistent with what we found. Additionally, one of our subjects had a bicuspid aortic valve, which is known to be accompanied by ascending aorta dilation, most often of a mild degree, in pediatric patients.13 Therefore, our findings of aortic dilation in only two subjects is no different from that in the general population.
The strength of the association of aortic dilation with cEDS and hEDS is questionable. This association was first reported by Leier et al. in their 1980 study of 19 patients; six of whom had aortic dilation, two of which were in the presence of a bicuspid aortic valve.6 A subsequent case series reported aortic dilation in five patients with cEDS and hEDS.14 More recently, in the largest study to date of 325 patients evaluated at Cincinnati Children’s Hospital Medical Center, which included patients from two prior studies9,10 in addition to new patients, Ritter et al. reported a prevalence of 14% for aortic dilation with a Z-score ≥2. In that study, 5.5% had an aortic Z-score ≥3.15 Similar to our results, the authors found there was no increase in the aortic Z-scores over time; in fact, they found the aortic Z-scores decreased over time. Further, the authors reported significant variance in the aortic Z-score depending on which Z-score database was used for the calculations. The investigators determined that “routine echocardiograms may not be warranted for pediatric patients with hEDS.” Asher et al. arrived at a similar conclusion in their study of 209 adults with hEDS.16 The investigators found aortic root dilation in 1.6% of patients, consistent with the expected prevalence in the general population. Additionally, none of the patients in their study had aortic dilation of a size to necessitate intervention. Those authors concluded routine echocardiograms to assess for aortic root dilation “may not be necessary unless warranted by presence of symptoms or family history.” Lastly, the recent study by Rauser-Foltz et al. identified aortic enlargement in none of the 208 pediatric patients with hEDS in their cohort. Thus, our findings regarding the aorta and the lack of significance of dilation in the setting of cEDS and hEDS are consistent with those of prior recent investigators, and our results are backed by the strength of strict adherence to the 2017 diagnostic criteria.
Aortic dilation is a common feature in multiple connective tissue disorders, such as Marfan syndrome (MFS),17 Loeys–Dietz syndrome (LDS),18 and 7q11.23 duplication syndrome,19 and represents a leading cause of morbidity and mortality for several. Given the commonality of aortic dilation in patients with connective tissue disorders, since EDS represents the most common connective tissue disorder, the suspicion that aortic involvement might be present in patients with EDS is understandable. However, the underlying biochemical pathological etiologies of disorders like MFS and LDS are entirely different from those of EDS. In both MFS and LDS, the underlying pathophysiologic mechanisms are related to abnormalities in TGF-β signaling, which impacts the integrity of the aortic wall resulting in aortic dilation.20,21 Conversely, cEDS and hEDS result from known or presumed genetic variants that impact the structure and function of collagen, including reduced levels of Tenascin-X, a protein involved in collagen fibrillogenesis, in patients with hEDS.1,22,23 Therefore, with varying underlying biochemical mechanisms affecting entirely different structural proteins, similar impacts on the aorta should not be assumed or expected.
Our results clearly demonstrate that aortic dilation is not a part of the clinical picture in cEDS and hEDS, as it did not occur more frequently than would be expected in the general population. Additionally, it is important to highlight that there was no correlation between patient age and aortic Z-scores in our cohort, even during the period of rapid growth velocity typically seen during puberty. This is in contrast to what is observed in patients with MFS and LDS, where aortic dilation increases with age. This reiterates the dissimilarity in aortic pathology between MFS or LDS and cEDS or hEDS. Further, prior studies have shown that patients with cEDS or hEDS who have normal aortas in childhood do not develop aortic dilation in adulthood,9 another important distinction from patients with MFS or LDS.17
Mitral valve prolapse was neither overly common nor particularly striking in our study cohort. Only four subjects had greater than trivial mitral valve prolapse, all of which were mild, and mitral regurgitation of greater than trivial severity was identified in only one subject. Our results are similar to those of other investigators. Rauser-Foltz et al. recently reported mitral valve prolapse in 1.3% of patients with confirmed or suspected hEDS and cEDS.24 In their study of 252 patients, Atzinger and colleagues reported mitral valve prolapse in 6% of patients, only one of which was greater than mild in severity.9 This prevalence was similar to the 6.4% reported by Asher et al. in their study of adults.16 Based on our results and some of these prior studies, mild mitral valve prolapse appears to be slightly more prevalent in cEDS and hEDS than the reported 2.4% in the general population.25 However, none of these studies have reported mitral valve prolapse of clinical significance. Thus, routine echocardiograms to assess for the presence of mitral valve prolapse do not appear to be of clinical benefit in patients with cEDS or hEDS.
Though our study has multiple strengths and important conclusions, there are limitations to the study that must be considered. This study was a retrospective review and is subject to the limitations inherent in such a design. By adhering strictly to the 2017 diagnostic criteria, we removed over 80% of patients in our institution who were suspected to have either cEDS or hEDS, which may have resulted in differences in the prevalences we report when compared with other studies. A similar loss of those diagnosed with cEDS and hEDS was reported by Asher et al. who found a 90% decrease in those diagnosed with hEDS after the introduction of the 2017 diagnostic guidelines.16 However, our goal was to characterize the cardiovascular findings in patients with a confirmed diagnosis of either cEDS and hEDS. Therefore, we see this as less of a limitation and more of a strength of the study. Additionally, we suspect that if the 2017 diagnostic criteria were applied to the subjects from other studies, as was the case in the study by Asher and colleagues, there would be a dramatic decline in the reported prevalence of aortic enlargement or valvular abnormalities. We did not have longitudinal follow-up echocardiograms in many of the study subjects, which could impact our conclusions about the lack of evidence supporting echocardiograms. Our clinical approach is not to perform follow-up echocardiograms in patients who do not have cardiovascular abnormalities. Nevertheless, our findings are in alignment with multiple studies that have had a greater degree of longitudinal follow-up, including the study by Asher et al. in adults.16 Finally, our cohort included only 12 subjects with cEDS, which may limit the generalizability of our findings. Our finding that 0/12 cEDS subjects had aortic dilation is in contrast to the recent report by Rauser-Foltz et al., who found that 7/16 pediatric cEDS subjects in their study had aortic dilation, although notably three of those patients also had bicuspid aortic valve.24 This discrepancy may be, in part, due to the small sample size in both studies, necessitating future multicenter studies to clarify the prevalence of aortic involvement in cEDS.
In conclusion, aortic dilation and/or valvular abnormalities are uncommon in pediatric patients with cEDS or hEDS, and when present they are not of clinically significant severity. None of the patients in our cohort with or without mild aortic or valvular anomalies required medication therapy or surgery, and none have had any cardiovascular events. When considered in the context of prior studies, our data suggest that routine echocardiographic evaluation is unwarranted for patients with cEDS or hEDS. Rather, complete cardiovascular evaluation, including imaging, should be limited to patients with concerning clinical symptoms, a family history of aortic enlargement or dissection/rupture, or for whom the diagnosis could potentially be another form of EDS such as cardiac–valvular or vascular EDS.
References
Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers–Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8–26.
McKusick VA. The Ehlers–Danlos syndrome. Heritable disorders of connective tissue. 4th ed. St. Louis: CV Mosby; 1972. p. 292–371.
Beighton P, de Paepe A, Danks D, et al. International nosology of heritable disorders of connective tissue, Berlin, 1986. Am J Med Genet. 1988;29:581–594.
Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers–Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers–Danlos National Foundation (USA) and Ehlers–Danlos Support Group (UK). Am J Med Genet. 1998;77:31–37.
Cabeen WR Jr, Reza MJ, Kovick RB, Stern MS. Mitral valve prolapse and conduction defects in Ehlers–Danlos syndrome. Arch Intern Med. 1977;137:1227–1231.
Leier CV, Call TD, Fulkerson PK, Wooley CF. The spectrum of cardiac defects in the Ehlers–Danlos syndrome, types I and III. Ann Intern Med. 1980;92 (2 Pt 1):171–178.
Dolan AL, Mishra MB, Chambers JB, Grahame R. Clinical and echocardiographic survey of the Ehlers–Danlos syndrome. Br J Rheumatol. 1997;36:459–462.
McDonnell NB, Gorman BL, Mandel KW, et al. Echocardiographic findings in classical and hypermobile Ehlers–Danlos syndromes. Am J Med Genet A. 2006;140:129–136.
Atzinger CL, Meyer RA, Khoury PR, Gao Z, Tinkle BT. Cross-sectional and longitudinal assessment of aortic root dilation and valvular anomalies in hypermobile and classic Ehlers–Danlos syndrome. J Pediatr. 2011;158:826–830.e1.
Wenstrup RJ, Meyer RA, Lyle JS, et al. Prevalence of aortic root dilation in the Ehlers–Danlos syndrome. Genet Med. 2002;4:112–117.
Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495.
Page Children’s Hospital Boston. Z-score calculator. https://zscore.chboston.org. Accessed 27 August 2019.
Ward RM, Marsh JM, Gossett JM, Rettiganti MR, Collins RT 2nd. Impact of bicuspid aortic valve morphology on aortic valve disease and aortic dilation in pediatric patients. Pediatr Cardiol. 2018;39:509–517.
Tiller GE, Cassidy SB, Wensel C, Wenstrup RJ. Aortic root dilatation in Ehlers–Danlos syndrome types I, II and III. A report of five cases. Clin Genet. 1998;53:460–465.
Ritter A, Atzinger C, Hays B, et al. Natural history of aortic root dilation through young adulthood in a hypermobile Ehlers–Danlos syndrome cohort. Am J Med Genet A. 2017;173:1467–1472.
Asher SB, Chen R, Kallish S. Mitral valve prolapse and aortic root dilation in adults with hypermobile Ehlers–Danlos syndrome and related disorders. Am J Med Genet A. 2018;176:1838–1844.
van Karnebeek CD, Naeff MS, Mulder BJ, Hennekam RC, Offringa M. Natural history of cardiovascular manifestations in Marfan syndrome. Arch Dis Child. 2001;84:129–137.
MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys–Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587.
Zarate YA, Lepard T, Sellars E, et al. Cardiovascular and genitourinary anomalies in patients with duplications within the Williams syndrome critical region: phenotypic expansion and review of the literature. Am J Med Genet A. 2014;164A:1998–2002.
Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366:1965–1976.
Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798.
Yamada K, Watanabe A, Takeshita H, et al. Measurement of serum Tenascin-X in joint hypermobility syndrome patients. Biol Pharm Bull. 2019;42:1596–1599.
Petersen JW, Douglas JY. Tenascin-X, collagen, and Ehlers–Danlos syndrome: tenascin-X gene defects can protect against adverse cardiovascular events. Med Hypotheses. 2013;81:443–447.
Rauser-Foltz KK, Starr LJ, Yetman AT. Utilization of echocardiography in Ehlers–Danlos syndrome. Congenit Heart Dis. 2019;14:864–867.
Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7.
Acknowledgements
S.L.P. acknowledges funding from the National Heart, Lung, and Blood Institute (K08HL148553).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Paige, S.L., Lechich, K.M., Tierney, E.S.S. et al. Cardiac involvement in classical or hypermobile Ehlers–Danlos syndrome is uncommon. Genet Med 22, 1583–1588 (2020). https://doi.org/10.1038/s41436-020-0856-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-0856-8
Keywords
This article is cited by
-
Echogenomics: Echocardiography in Heritable Aortopathies
Current Cardiology Reports (2024)
-
Bicuspid Aortic Valves: an Up-to-Date Review on Genetics, Natural History, and Management
Current Cardiology Reports (2022)