Abstract
Purpose
This study sought to determine genetics and oncology specialists’ views of integrating BRCA1 and BRCA2 testing in epithelial ovarian and breast cancer into routine practice.
Methods
Qualitative interviews were designed using the Consolidated Framework for Implementation Research. Questions included experiences or views of the BRCA testing processes, implementation needs of oncology health professionals, perceived challenges, and future ideas for interventions to integrate genetic testing into oncology.
Results
Twenty-two participants were interviewed from twelve health organizations and four themes were identified: (1) embracing the shift to mainstream genetic testing, with the majority of participants viewing BRCA testing as clinically useful and routine use important for maintaining a patient centered process; (2) the need for communication networks and role delineation to integrate routine genetic testing; (3) factors that influence sustaining routine genetic testing, including ongoing training, resources and funding, real-world adaptation, system complexity, and champions; and (4) variation in system interventions for integrating routine genetic testing align to organizational context.
Conclusion
Findings illustrate the need for integrating genetic testing into routine oncology, and that adaptation of interventions and processes is essential to sustain a feasible model. An understanding of individual and organizational implementation factors will help to prepare for future integration of routine genetic testing in other cancers.
Similar content being viewed by others
INTRODUCTION
Fifteen to twenty percent of high-grade serous ovarian cancers have been shown to harbor BRCA variants.1 Recommendations made in the United States in 2007 allow for all epithelial ovarian cancer (EOC) patients to have BRCA testing regardless of age or family history.2 In spite of this recommendation, a suboptimal referral rate to genetic counseling (GC) for women with EOC of 30% exists internationally.3,4,5,6 In Australia, eviQ (a consensus evidence base for cancer treatment and information) recommends that BRCA testing be considered for patients with triple negative breast cancer under 50 years and EOC under 70 years or at any age with a significant family history of breast or ovarian cancer in a close relative or relapsed EOC.7 The Australian government has publicly funded BRCA1 and 2 testing of eligible candidates since November 20178 and this change in testing policy allows for multiple breast or ovarian cancer genes (BRCA1, BRCA2, PALB2, STK11, PTEN, CDH1, and TP53) to be tested9 by any medical specialist—this is known as mainstreaming.10 Mainstreaming of genetic testing requires a shift in practice from referral of patients to a specialist genetics service for GC and genetic testing to now be facilitated by their oncology health professional (OHP). The direct integration of genetic testing through the oncology setting has improved access to testing and increased the identification of BRCA individuals for effective cancer prevention to be promoted.11,12,13 However, the implementation and contextual factors at an organizational and individual level that lead to successful BRCA test integration in some settings are unknown.
Integrating BRCA testing for EOC allows the targeting of therapy through the use of poly ADP ribose polymerase inhibitors (PARPi) in BRCA pathogenic variant positive EOC individuals. In 2017, PARPi were included on the Australian Register of Therapeutic Goods for patients with platinum-sensitive relapsed BRCA pathogenic variant high-grade serous EOC and Medical Benefit Scheme (MBS) funding for any medical specialist to order BRCA testing for these women. Additionally, women with EOC and a 10% probability of having a variant in BRCA1, BRCA2, PALB2, STK11, PTEN, CDH1, and TP53 have access to testing funded under MBS9 and this has led to a shift in pretest GC responsibilities onto the OHP, requiring the oncology workflow system to accommodate the process of genetic testing and delivery of results.
Genetic test integration into routine oncology practice assumes that the OHP will take on the role of pre-test information and informed consent for BRCA testing. BRCA mainstreaming programs introduced into the United Kingdom, United States, and Australia11,12,13 have identified interventions that help to integrate genetic testing into oncology processes. Interventions such as the upskilling of nongenetics health professionals through cancer genetics education11,12,13 and obtaining consent for genetic testing,11 embedding a genetic counselor,12 and use of the electronic system to streamline the process13 were identified as important components of the mainstreaming toolkits. Specifically, these programs found an increase of BRCA testing completion rate from 27% to 82%13 and 54% to 90% compared with baseline12 and 100% testing completion rate.11 The mainstreaming toolkit for piloting BRCA mainstreaming in various hospitals in Australia (Fig. S1) encompasses strategies such as education and obtaining consent with a standard form and pathology request. The assessment effect of these strategies at improving genetic testing access and completion is underway (manuscript under review); however, evaluation of contextual (organization culture, structure, and implementation climate) and individual provider issues (readiness to implement and individual needs) that impact on implementation efforts across multiple hospital sites was not included. This implementation science lens evaluation is required to inform strategy decisions to support future implementation efforts in BRCA mainstreaming. Another benefit of such evaluation gives generalizable lessons for wider integration of genetic testing in oncology.
The mainstreaming programs and strategies described above were not underpinned with an implementation science framework or lens to evaluate or guide the intervention design and implementation efforts taking into account the organizational context. Implementation frameworks enhance the translation of new evidence into clinical practice by giving a better understanding of how and why implementation succeeds or fails.14 The Consolidated Framework for Implementation Research (CFIR) encompasses 39 constructs in five domains that address dissemination, innovation, organizational change, implementation, knowledge translation, and research uptake.15 It has been widely used across various disciplines and health organizations to evaluate pre- and postimplementation efforts and implementation processes in real-world settings.16 As various approaches are being trialed to integrate genetic testing into oncology, it is important to consider the implementation factors of GC pre-test role acquisition by OHP and to understand their service and support needs in the organizational context. In light of these developments, this study sought to determine genetics and oncology specialists’ views of integrating BRCA testing in EOC and breast cancer into routine oncology practice using an implementation science framework to examine the successes and challenges in various Australian hospitals.
MATERIALS AND METHODS
Participants
Purposive sampling was used to gather evidence of implementation factors within different hospital organizations by sending email invitations to the heads of Australian Familial Cancer Clinics (FCC) and oncology, the Australian Genetic Testing Mainstreaming Collaborative Group, and trainee radiation and medical oncologists as part of the Basic Sciences in Oncology program in Australia to participate in a semistructured telephone interview. The invitation included a snowball sampling approach to forward the study invitation to potentially knowledgeable interviewees such as oncology or genetics colleagues who had experience or views about mainstreaming of genetic testing in ovarian and breast cancer either within or outside of their organization.
Procedure
The development of the interview guide was devised using CFIR’s five domains: intervention characteristics, outer setting, inner setting, characteristics of the individuals involved, and the process of implementation. Intervention characteristics are key attributes of interventions that influence the success of implementation.15 The outer and inner setting describe factors that influence implementation from an economic, political, and social context in the outer setting to structural, political, and cultural contexts within organizations in the inner setting.15 The characteristics of individuals describes individuals involved with the intervention and/or implementation process.15 The process of implementation connects an intervention with its setting to understand the process of effective implementation.15 All CFIR domains and the associated 90 possible questions15 were included in the initial draft of the interview guide. Each member of the Australian Genetic Testing Mainstreaming Collaborative Group (senior genetic counselors) were emailed the initial copy of the interview guide and asked which questions to include or not under the five CFIR domains. Their responses were compared with those of the study authors through Excel version 10 (2016), which led to refinement and selection of 24 qualitative questions. A consensus on the four key contextual implementation factors was reached through discussion and led to a further refinement of 17 questions to include and facilitate data collection on (1) oncology and genetic health professionals' (GHPs) views about the success and challenges of BRCA mainstreaming, (2) the support and education needs of OHPs for sustaining mainstreaming of genetic testing, (3) the readiness of OHPs to implement mainstreaming genetic testing, and (4) organizational and individual factors that will facilitate or hinder implementing and sustaining mainstreaming. The final interview guide consisted of 17 questions across four CFIR domains (Supplementary Material), which elicit theoretically underpinned data on the key factors affecting the implementation process of BRCA mainstreaming. Email contact was initiated with all consenting participants to arrange a telephone interview and all interviews were conducted between March and September 2019 by a single researcher (R.O.S.). This study received ethics approval from the University of Sydney’s Human Research Ethics Committee (approval number 2018/973).
Data analysis
Interviews were transcribed, de-identified, and coded in an iterative manner to identify common themes using a researcher-generated coding tree. The codes were created by analyzing the raw interview data and were developed (by R.O.S.) using the first interview transcript and mapped to the CFIR domains. The next two transcripts were independently coded by three researchers (R.O.S., N.T., N.M.R.) and checked for concordance. A consensus agreement on discordance codes was reached between the independent coders, giving an overall concordance rate of 80%. Refinement of the coding occurred at each of these discussions. Counting of codes allowed visualization of patterns and similarities in the data, leading to thematic characterizations.17 The remaining transcripts were analyzed using the established codes and an inductive thematic approach18 using the grounded theory methods of coding and constant comparison19 to identify themes encompassed within the data. Final thematic consensus was reached through research team meetings.
RESULTS
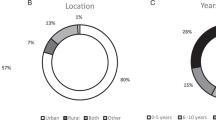
Twenty-four participants consented and 22 were interviewed and two indicated they were no longer available. Participation represented all the Australian states with the exception of Tasmania (Table 1). Participants included genetic counselors and clinical geneticists (GHPs), nurses, a surgeon, and oncologists (OHPs). The majority of participants (91%; 20/22) had more than 5 years’ experience in practice and 9% (2/22) had 1–5 years in practice with five GHPs and OHPs holding senior or leadership positions, respectively.
Implementation influences of inner, outer setting and implementation process
Embracing the shift to mainstreaming of genetic testing
The majority of participants (18/22; 8 GHPs and 10 OHPs) indicated that they value mainstreaming the genetic testing process to give patients further options in their treatment for EOC (clinical utility) and facilitate a streamlined process. Public and private metropolitan hospital based (13/18) genetic counselors (3/6), consultant geneticists (2/2), oncologists (5/7), and nurses (3/7) expressed clinical utility as important (Table S5). Although many participants reported that current mainstreaming processes are streamlined and provide better access for patients, there was a recognized need for optimization, especially in regard to results delivery and follow up (14/22; 7 GHPs and 7 OHPs). This subtheme was expressed by genetic counselors (5/6) from metropolitan, statewide, and regional services and oncologists (6/7) and nurses (5/7) in public metropolitan and regional or community hospital settings (Table S5). Most participants (12/22; 5 GHPs and 7 OHPs) expressed the importance of a patient centered process through continued contact with the OHP and understanding the right time to introduce genetic testing into the EOC care process. Public metropolitan hospital based (8/18) genetic counselors (2/6), consultant geneticists (1/2), oncologists (3/7), and nurses (2/7) and all genetic counselors (2/2) and oncologists (2/2) in a regional and statewide setting viewed the patient centered process as important (Table S5). OHPs were viewed as the most appropriate to facilitate routine genetic testing in a patient centered manner. Illustrative quotes in Tables 2 and S1 relate to the various subthemes and map to either the inner (relative priority construct) or outer setting (patient needs) and implementation process (executing) domains of CFIR.
Implementation influences of inner setting and individuals
Communication networks and role delineation needed to integrate genetic testing
Most participants (12/22; 8 GHPs and 6 OHPs)—mainly genetic counselors (3/6), consultant geneticists (2/6), oncologists (3/7), and nurses (3/7) based in metropolitan public and private hospitals (11/18) with a smaller number in statewide and regional settings (3/6 genetic counselors and 1/7 oncologists) (Table S5)—expressed the importance of good communication between genetics and oncology. Communication networks and collaboration to ensure continued support for integrating genetic testing through a point of contact in genetics (15/22; 7 GHPs and 8 OHPs) were mentioned by genetic counselors (3/6), consultant geneticists (2/2), oncologists (4/7), and nurses (2/7) based in metropolitan public and private hospitals (11/18) and genetic counselors (2/2) and oncologists (2/2) in statewide and regional settings (4/4) (Table S5). Both oncology and genetics participants indicated the need for role delineation (15/22; 8 GHPs and 7 OHPs) and to have a clearer pathway of responsibility for different aspects of the patientʼs care continuum. Genetic counselors (3/6), consultant geneticists (2/2), oncologists (3/7), and nurses (3/7) based in metropolitan public and private hospitals (11/18) and all genetic counselors and oncologists in statewide and regional settings (2/2) viewed role delineation as important (Table S5). Illustrative quotes in Tables 3 and S2 relate to the various subthemes and map to either the inner setting (networks and communication) or individuals involved (other personal attributes) domains of CFIR.
Implementation influences of inner setting, intervention characteristics, and process
Influencing factors on sustaining routine genetic testing
Almost all participants (18/22; 8 GHPs and 10 OHPs) had views on ongoing training in genetics to sustain mainstreaming in practice and enabling upskilling to increase comfort levels with the pre-test GC role. Public and private hospital based metropolitan (13/18) genetic counselors (3/6) and consultant geneticists (2/2), oncologists (5/7), and nurses (3/7) and all regional and statewide participants expressed ongoing training as important (Table S5). Some highlighted the need for training in genetics to be a core component of the university curricula of nursing and oncology trainees (7/22; 4 GHPs and 3 OHPs). Most participants (13/22; 6 GHPs and 7 OHPs) identified sufficient resources and funding as a barrier to sustaining mainstreaming in public and private hospital based metropolitan (11/18) settings, with genetic counselors (3/6) and consultant geneticists (2/2), oncologists (4/7) and nurses (2/7), and some in regional and statewide participants raising this as a barrier (Table S5). Many participants (12/22; 6 GHPs and 6 OHPs) talked about the real-world adaptation of the pilot research mainstreaming project (Fig. S1) with varying degrees of success and challenges to embed mainstreaming into routine practice in public and private metropolitan hospital based (7/18) settings, with genetic counselors (1/6) and consultant geneticists (2/2), oncologists (2/7), and nurses (2/7) and all participants in regional and statewide settings indicating that testing was in routine practice or still being piloted (Table S5). Illustrative quotes in Tables 4 and S3 relate to the various subthemes and map to either the inner setting (readiness for implementation—access to knowledge and information or available resources) or intervention characteristics (adaptability) domains of CFIR.
Some participants (7/22; 5 GHPs and 2 OHPs) commented on the complexities of private and public hospital processes for follow up and tracking of results due to the lack of a shared information system. Public and private hospital based metropolitan (4/18) genetic counselors (2/6), consultant geneticists (1/2), and nurses (1/7), as well as genetic counselors (1/2) and oncologists (2/2) in regional and statewide settings, expressed challenges with integrating public and private systems for sharing genetic information (Table S5). Some participants (5/22; 3 GHPs and 2 OHPs) raised concerns due to complications of genetics not being integrated into the main hospital public setting—genetic counselors (1/6), consultant geneticists (1/2), and oncologists (2/7) and a genetic counselor (1/2) in the statewide system (Table S5)—with concerns regarding privacy of genetic information noted. Most participants (13/22; 7 GHPs and 6 OHPs) spoke about the importance of having a genomics “champion” within the oncology team or department. Public and private hospital based metropolitan genetic counselors (3/6), consultant geneticists (2/2), oncologists (3/7) and nurses (2/7) and genetic counselors (2/2) and one oncologist in the statewide and regional setting expressed the importance of champions to maintain routine use of genetic testing within the oncology service. In settings where there was no existing champion to influence oncology practices, there were challenges to implementation and other system structures limiting the generalizability of integrating a genetic testing pathway. Illustrative quotes in Tables 4 and S3 relate to the various subthemes and map to either the inner setting (readiness for implementation and implementation climate) or intervention characteristics (adaptability) or process (engaging champions) domains of CFIR.
Implementation influences of intervention characteristics
System interventions for integration of genetic testing
Most participants (14/22; 7 GHPs and 7 OHPs) were supportive of having a genetic counselor embedded in an oncology clinic as a version of mainstreaming. Public and private hospital based metropolitan (10/18) genetic counselors (2/6) consultant geneticists (2/2), oncologists (3/7), and nurses (3/7) and all regional and statewide participants expressed embedding a genetic counselor into the oncology setting as a potential intervention to support mainstreaming (Table S5). However, a few participants (3/22; 1 GHPs and 2 OHPs) felt that the funding and structure of certain state health systems would limit it as a generalizable intervention. Some participants (7/22; 1 GHPs and 6 OHPs) viewed multi disciplinary team (MDT) meetings with documentation and tracking of genetic testing outcomes in the existing template within the public hospital metropolitan setting (6/18) from the oncologist (3/7), nursing (2/7), and genetic counselor (1/6) perspective (Table S5) as a suitable intervention for their system. Others (9/22; 3 GHPs and 6 OHPs) expressed that a centralized or main electronic tracking system in the medical record would be a more suitable system intervention for public hospital metropolitan (8/18) based genetic counselors (2/6) and consultant geneticists (1/2), as well as from the oncologist (4/7) and nursing perspective (1/7). Some participants (6/22; 2 GHPs and 4 OHPs) suggested a flowchart or checklist intervention to ensure that there is an understandable pathway for OHPs to follow for incorporating genetic testing into their routine practice from the public hospital metropolitan (5/18) based genetic counselor (1/6), consultant geneticist (1/2), oncologist (1/7), and nursing perspective (2/7). Online training, automatic or email reminders, information provision, applications, and telehealth (1–3 participants) were other suggested interventions to facilitate mainstreaming within various hospital settings per role of the participant (Table S5). Illustrative quotes for each intervention are provided in Tables 5 and S4 and relate to the intervention characteristics domain of CFIR.
DISCUSSION
This study explored current and emerging integration of BRCA germline genetic testing for EOC and subsets of breast cancer in various health settings in Australia. Overall, both OHP and GHP recognize the value of routine BRCA testing to inform treatment management decisions and to maintain patient centered care through the ongoing relationship with the OHP. Most organizations had to adapt their processes to ensure incorporating BRCA testing was a suitable fit with their patients and systems. Collaboration, communication, and role delineation between OHP and GHP and departments were viewed as important in initiating and sustaining routine testing. Ongoing training, additional resources, funding, and mainstreaming champions, especially in settings where genetic testing was not wholly integrated into routine practice, were recognized modes of facilitating or sustaining the integration of BRCA testing. Generalizable interventions to facilitate routine genetic testing were accepted as positive practice, such as embedding genetic counselors into oncology departments, working better with MDT, and electronic patient tracking through medical records to ensure results were followed up. However, organizational variation existed as to what intervention would suit particular systems to facilitate and sustain a future model for genetic test adoption.
The survival advantage of those with EOC pathogenic variants targeted with PARPi20 drives the initiation of routine BRCA testing, improving access and personalized medicine approaches. Our findings show both OHP and GHP view routine BRCA testing for EOC clinically useful in that testing was streamlined and delivered further treatment options while maintaining personalized care for their patients. Oncologists viewed the perceived relative advantage and clinical utility of routine BRCA testing as important in their practice for treatment options in other BRCA mainstreaming programs.11,12,21 Clinical utility in oncology and neurology in the United States allowed a streamlined approach and was preferred in all specialties.22 Surgeons and oncologists in the United Kingdom viewed a simplified pathway for mainstreaming as efficient for turnaround and expediting treatment decisions.23 Our study found that OHPs were viewed as the most appropriate professional to take on the role of pre-test GC for routine BRCA testing and adhere to a patient centered process. Similarly, the oncologist was viewed as key to providing patient follow up for integrating universal tumor screening (UTS) in colorectal cancers and in routine genetic testing for women with breast cancer due to the ongoing patient–practitioner relationship.23,24 Our findings support previous research and show that implementation factors in the inner, outer, and process domains such as relative priority, executing, and patients’ needs appear to drive the uptake and sustainability of integrating genetic testing into routine care.
The adoption of genomics is an acknowledged challenge in health systems due to the gap between the fast pace of genomic discoveries and their translation into clinical care, even when there is proven validity and utility.25,26 In view of the recognized challenges, our results indicate that role delineation, collaboration, and communication are important implementation factors for ensuring OHPs feel supported in taking on the role of pretest GC. The Implementing Genomics in Practice (IGNITE) Network in the United States showed that effective communication and collaboration factor into the successful integration of genomics across specialities.27 Successful implementation factors such as educational support and pathways to routine genetic testing with partnership from genetics and oncology are highlighted in EOC mainstreaming programs internationally.11,12,13 Delineating clinical responsibility with OHPs responsible for pathology evaluation and treatment management decisions and embedding a genetic counselor responsible for pre-test GC during chemotherapy sessions and genetic test result follow up in the oncology workflow were described as success factors in one approach to integrating BRCA testing into oncology in Australia.12 Our findings support previous research and illustrate that implementation factors in the inner and individual domains such as collaboration, role delineation and, good communication between OHP and GHP influence implementation success in the adoption of routine genetic testing.
Mainstreaming is challenged by the recognized need to upskill medical specialists with the relevant capability to take on the new role of pre-test GC.28 The most common barrier to access to genetic testing identified by patients was that their doctor did not recommend it or physicians felt unprepared to use genetic information in their practice.29,30 Most participants in our study recognized the need for ongoing training to support OHPs in adopting the role of pre-test GC and some indicated that for long-term sustainability, genetics education needs to be part of university training curricula for doctors and nurses. The need to keep abreast of the evolution of knowledge in genomics and oncology due to new treatments and testing regimes requires the cancer workforce to be continually updated as new genes are discovered, variants are interpreted, and tumor testing evolves.31 Our results indicate that the integration of genetic testing would be influenced by an inner setting implementation factor in the organization's readiness for implementation through long-term access to knowledge and information to upskill in genetics.
Additional issues were found that highlight implementation factors that extend beyond ongoing training and upskilling of OHPs in cancer genetics. These issues that would impact sustainability of mainstreaming include ensuring the availability of sufficient infrastructure resources and funding for personnel, and managing the differing needs of public and private information systems to store and allow access to genetic information in the main health record. Additional infrastructural resources, staff capacity, and time commitment are also seen as barriers in other studies.23 Systems infrastructure with lack of a tracking system for genetic referrals and patient follow up in 33% of community based hospitals incorporating UTS32 and laboratory processing and systems for electronic ordering and tracking for UTS33 were identified as key implementation success factors. Our findings support previous research and illustrate that implementation factors such as readiness for implementation through available resources, infrastructure, and funding influence sustainability, and that the mainstreaming interventions adaptability and compatibility impact the real-world adoption of routine genetic testing.
The importance of identifying champions to influence adoption of interventions and innovations in health systems is recognized as an important implementation science factor.34 Our findings suggest the need for genomics champions to push for routine incorporation of genomics into oncology. This is echoed in other studies that identified the need to have local champions to influence adoption of UTS in colorectal cancer and incorporate treatment focused genetic testing in routine oncology for breast cancer,23,24,33 along with the need for mainstreaming champions to provide leadership to influence the adoption of genomics.23,35 Due to the ongoing need for education and champions, suitable health professionals with specialist genetic knowledge such as genetic counselors or OHP genomics champions are required to ensure that evolving evidence is translated into clinical practice. Our findings on suitable interventions for integrating genomics in oncology suggest that genetic counselors could facilitate the role of mainstreaming champions to allow for real-world adoption of genomics in cancer care.
Some participants suggested that embedding a genetic counselor (either for a transitional period or more long-term if funding resources allow) as an intervention would be useful for future sustainability to support routine integration of genetic testing into oncology, which was an approach employed in one Australian program.13 However, this approach is demanding on the resources of genetic services, and would not be an option for some service structures (including some statewide or smaller rural services) and may limit the upskilling of OHPs to take on the role of pretest GC. Some strategies to consider these demands for future integration of testing could include different models of GC that move away from the traditional face-to-face hospital based model to alternatives such as telephone or telemedicine GC36,37 or pretest education provision through written (pamphlets, booklets) and online platforms (websites, decision aids).38,39
The other suggested interventions varied between organizations such as MDT meetings and template documents to include tracking of patients who had BRCA testing, a centralized or main electronic tracking system in the medical record, a flowchart or checklist intervention to ensure that there is an understandable mainstreaming pathway, online training, automatic or email reminders, information provision, electronic applications, and telehealth to facilitate integrating routine genetic testing within different hospital settings. Successful international health system interventions to date include online education and upskilling of nongenetics health professionals in cancer genetics and consenting for GT, the development and testing of a care and referral pathway for the collaborative working of the oncology and genetic specialist, utilization of the electronic health record, and streamlined patient appointment services for universal referral to GC.11,12,13 The varied views on intervention characteristics' suitability and need for adaptation to the BRCA mainstreaming EOC intervention provided (Fig. S1) suggest that preimplementation research and evaluation of implementation is required to allow for ease of adoption and sustainability of BRCA mainstreaming intervention. Our findings suggest that the key attributes of the BRCA mainstreaming intervention characteristics are important to evaluate in pre-implementation research to ensure compatibility of the interventions at the individual organizational level.
It is broadly recognized that pre-implementation research, to examine the chances of an intervention working in real-world settings, does not always occur.40 As BRCA and other genetic testing integrates into routine oncology care, pre-implementation research to evaluate the organization context and process to suitably design and adapt multiple hospital system interventions before implementation would aid the effectiveness and sustainability of integrating routine genetic testing in other cancer types and across a diverse set of organizations and structures.
This study has a number of strengths and limitations. We were able to collect data from health-care professionals from all Australian states involved in mainstreaming. Not all professional groups were represented, with a lack of breast surgeons’ involvement; however, an equal number of genetics and oncology professionals were represented. Most participants had considerable experience in health-care practice and had or were in a variety of roles in the mainstreaming process; however, as not all participants had direct experience of current mainstreaming pathways, their views may not be representative of all GHPs and OHPs in Australia. A diverse set of health organizations and systems were included, leading to generalizable genomics implementation lessons for metropolitan, rural, and statewide health systems. R.O.S. was the sole interviewer and is a qualified genetic counselor, leading to consistency in data collection and interpretation. To overcome any bias of data analysis and reporting due to one genetic counselor's perspective, the data were independently assessed by implementation scientists (N.T. and N.M.R.) to ensure objectivity in reporting. The use of CFIR underpinned the collection of data and facilitated the interpretation of our findings to identify lessons and gaps in the needs of individuals and organizations in the implementation of routine oncology BRCA testing. Understanding the implementation factors that affect the adoption of routine BRCA testing across hospital organizations provides important information for future implementation research to guide and tailor implementation strategies for health-care organizations seeking to sustain models for integrating routine genetic testing in other cancer types.
References
Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663.
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology. Version 2.2013 Accessed 15 February 2014. https://www.nccn.org/professionals/physician_gls/default.aspx.
Metcalfe KA, Fan I, McLaughlin J, Risch HA, Rosen B, Murphy J, et al. Uptake of clinical genetic testing for ovarian cancer in Ontario: a population based study. Gynecol Oncol. 2009;112:68–72.
Meyer LA, Anderson ME, Lacour RA, Suri A, Daniels MS, Urbauer DL, et al. Evaluating women with ovarian cancer for BRCA and BRCA2 mutations: missed opportunities. Obstet Gynecol. 2010;115:945–952.
Petzel SV, Vogel RI, Bensend T, Leininger A, Argenta PA, Geller MA. Genetic risk assessment for women with epithelial ovarian cancer: referral patterns and outcomes in a university gynecologic oncology clinic. J Genet Couns. 2013;22:662–673.
Demsky R, McCuaig J, Maganti M, Murphy KJ, Rosen B, Armel SR. Keeping it simple: genetics referrals for all invasive serous ovarian cancers. Gynaecol Oncol. 2013;130:329–333.
eviQ. Genetic testing for heritable mutations in the BRCA1 and BRCA2 genes. Sydney, Australia: Cancer Institute NSW; 2017. https://www.eviq.org.au/Protocol/tabid/66/id/620/Default.aspx?popup=1.
Petelin L, James PA, Trainer AH. Changing landscape of hereditary breast and ovarian cancer germline genetic testing in Australia. Intern Med J. 2018;48:1269–1272.
Medical Service Advisory Committee (MSAC). 1411.1 Genetic testing for hereditary mutations predisposing to cancer (breast and/or ovarian) (resubmission). 2017. http://www.msac.gov.au/internet/msac/publishing.nsf/content/1411.1-public.
Kirk J, Barlow-Stewart KK, Poplawski NK, Gleeson M, Tucker K, Friedlander M. Medicare-funded cancer genetic tests: a note of caution. Med J Aust. 2018;209:193–196.
George A, Riddell D, Seal S, Talukdar S, Mahamdallie S, Ruark E, et al. Implementing rapid, robust, costeffective, patient-centred, routine genetic testing in ovarian cancer patients. Sci. Rep. 2016;6:1–8.
Uyar D, Neary J, Monroe A, Nugent M, Simpson P, Geurts JL. Implementing a quality improvement project for universal genetic testing in women with ovarian cancer. Gynecol Oncol. 2018;149:565–569.
Kentwell M, Dow E, Antill Y, Wrede CD, McNally O, Higgs E, et al. Mainstreaming cancer genetics: a model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol. 2017;145:130–136.
Nielsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53.
Damschroder L, Aron D, Keith R, Kirsh S, Alexander J, Lowery J. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;17:11:72.
Sandelowski M. Whatever happened to qualitative description?. Res Nurs Health. 2000;23:334–340.
Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27:237–246.
Strauss A, Corbin J. Basics of qualitative research: techniques and procedures for developing grounded theory. 2nd ed. Thousand Oaks, CA: Sage; 1998.
Norquist BM, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–490.
Percival N, George A, Gyertson J, Hamill M, Fernandes A, Davies E, et al. The integration of BRCA testing into oncology clinics. Br J Nurs. 2016;25:690–694.
Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16:238–245.
Hallowell N, Wright S, Stirling D, Gourley C, Young O, Porteous M. Moving into the mainstream: healthcare professionals’ views of implementing treatment focussed genetic testing in breast cancer care. Fam Cancer. 2019;18:293–301.
West KM, Burke W, Korngiebel DM. Identifying “ownership” through role descriptions to support implementing universal colorectal cancer tumor screening for Lynch syndrome. Genet Med. 2017;11:1236–1244.
Rogowski WH, Grosse SD, Khoury MJ. Challenges of translating genetic tests into clinical and public health practice. Nat Rev Genet. 2009;10:489–495.
Manolio TA, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267.
Orlando LA, Sperber NR, Voils C, Nichols M, Myers RA, Wu R, et al. Developing a common framework for evaluating the implementation of genomic medicine interventions in clinical care: the IGNITE Network’s Common Measures Working Group. Genet Med. 2018;20:655–663.
Duoma KFL, Smets EMA, Allain DC. Non-genetic health professionals’ attitude towards, knowledge of and skills in discussing and ordering genetic testing for hereditary cancer. Fam Cancer. 2016;15:341–350.
Kurian AW, Griffith KA, Hamilton AS, Ward KC, Morrow M, Katz SJ, et al. Genetic testing and counseling among patients with newly diagnosed breast cancer. JAMA. 2017;317:531–533.
Owusu Obeng A, Fei K, Levy KD, Elsey A,R, Pollin T,I, Ramirez AH, Weitzel K,W, Horowitz CR. Physician-reported benefits and barriers to clinical implementation of genomic medicine: a multi-site IGNITE Network survey. J Pers Med. 2018;8:24.
Williams MS. Early lessons from the implementation of genomic medicine programs. Annu Rev Genom Hum Genet. 2019;20:389–411.
Beamer LC, Grant ML, Espenschied CR, Blazer KR, Hampel HL, Weitzel JN, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–1063.
Schneider JL, Davis J, Kauffman TL, Reiss JA, McGinley C, Arnold K, et al. Stakeholder perspectives on implementing a universal Lynch syndrome screening program: a qualitative study of early barriers and facilitators. Genet Med. 2016;18:152–161.
Jenkins J, Calzone KA, Caskey S, et al. Methods of genomic competency integration in practice. J Nurs Scholarsh. 2015;47:200–210.
Crawford JM, Bry L, Pfeifer J, Caughron SK, Black-Schaffer S, Kant JA, et al. The business of genomic testing: a survey of early adopters. Genet Med. 2014;12:954–961.
Kinney AY, Steffen LE, Brumbach BH, Kohlmann W, Du R, Lee JH, et al. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-year follow up. J Clin Oncol. 2016;34:2914–2924.
Hilgart JS, Hayward JA, Coles B, Iredale R. Telegenetics: a systematic review of telemedicine in genetics services. Genet Med. 2012;14:765–776.
Lieberman S, Lahad A, Tomer A, Cohen C, Levy-Lahad E, Raz A. Population screening for BRCA1/BRCA2 mutations: lessons from qualitative analysis of the screening experience. Genet Med. 2016;19:628–634.
Sie AS, Spruijt L, van Zelst-Stams WA, et al. DNA-testing for BRCA1/2 prior to genetic counselling in patients with breast cancer: design of an intervention study, DNA-direct. BMC Womens Health. 2012;12:12
Rabin B, Glasgow RE. An implementation science perspective on psychological science and cancer: what is known and opportunities for research, policy, and practice. Am Psychol. 2015;70:211–220.
Acknowledgements
This research was funded by a Cancer Council New South Wales PhD scholarship and by a Translational Cancer Research Network Clinical PhD Scholarship Top-up award, supported by the Cancer Institute NSW supporting R.O.S. in the completion of her PhD studies in the Faculty of Medicine and Health at the University of Sydney.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
M.K., M.G., and K.M.T. received remunerations from AstraZeneca in the initial development of BRCA mainstreaming interventions for epithelial ovarian cancer. The other authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
O’Shea, R., Rankin, N.M., Kentwell, M. et al. How can Australia integrate routine genetic sequencing in oncology: a qualitative study through an implementation science lens. Genet Med 22, 1507–1516 (2020). https://doi.org/10.1038/s41436-020-0838-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-020-0838-x
Keywords
This article is cited by
-
Postgraduate training in Cancer Genetics—a cross-specialty survey exploring experience of clinicians in Ireland
Irish Journal of Medical Science (1971 -) (2022)
-
Stakeholders’ views of integrating universal tumour screening and genetic testing for colorectal and endometrial cancer into routine oncology
European Journal of Human Genetics (2021)