Abstract
Purpose
We sought to assess the readiness of the United Kingdom (UK) National Health Service to implement a Genomic Medicine Service. We conducted a systematic literature review to identify what is known about factors related to the implementation of genomic medicine in routine health care and to draw out the implications for the UK and other settings.
Methods
Relevant studies were identified in Web of Science and PubMed from their date of inception to April 2018. The review included primary research studies using quantitative, qualitative, or mixed methods, and systematic reviews. A narrative synthesis was conducted.
Results
Fifty-five studies met our inclusion criteria. The majority of studies reviewed were conducted in the United States. We identified four domains: (1) systems, (2) training and workforce needs, (3) professional attitudes and values, and (4) the role of patients and the public.
Conclusion
Mainstreaming genomic medicine into routine clinical practice requires actions at each level of the health-care system. Our synthesis emphasized the organizational, social, and cultural implications of reforming practice, highlighting that demonstration of clinical utility and cost-effectiveness, attending to the compatibility of genomic medicine with clinical principles, and involving and engaging patients are key to successful implementation.
Similar content being viewed by others
INTRODUCTION
Globally, health systems anticipate the incorporation of genomic medicine into clinical practice. In the United Kingdom (UK), the Genomic Medicine Service was launched in 2018, coinciding with the completion of the 100,000 Genomes Project. The service plans to mainstream genomic testing in the National Health Service (NHS), from single-gene to genome sequencing (GS), and, in the long term, expand the use of genomics beyond rare diseases and cancer.1 This vision was set out in the Chief Medical Officer’s 2017 report Generation Genome, which envisaged the imminent implementation of genomic medicine while acknowledging a need to consider infrastructural, workforce, ethical, and other issues raised by an expansion of this technology.2 Integrating genomic medicine poses considerable challenges to health-care organizations, however evidence to support implementation remains lacking.3 This review aims to identify what is known about factors related to the implementation of genomic medicine in routine health care and to draw out the key implications for the UK and other settings. We conducted a narrative synthesis to examine the complex organizational, social, and cultural factors involved in implementing genomic medicine.
MATERIALS AND METHODS
Literature search strategy
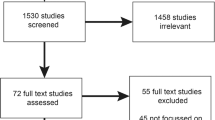
We conducted a systematic search for studies that described implementing genomics and genetics into clinical practice. Clinical applications of genomic medicine remain in the early stages; accordingly, our search included clinical genetics to examine the implications of this evidence for genomic medicine. Searches were conducted across two databases, Web of Science and PubMed, and included all literature in the databases from their date of inception to April 2018 (Fig. 1). The search strategy combined “genomics” or “genomic medicine” with terms such as “implementation” and “health services” (Supplementary Table S1).
Study selection criteria
The review included primary research studies and systematic reviews. The search was not limited to UK studies, or to countries with similar health-care systems, as we believed research findings from other settings might be instructive for the UK context. Articles were excluded if they were not written in English. Editorials, commentaries, conference abstracts, and methodological papers without presentation of research findings were excluded. Also excluded were papers with a molecular/biological focus, papers on peri- or prenatal genetics, and papers about genetics research rather than clinical practice. Additional papers were identified through hand-searching reference lists and citation tracking of included studies. After removing duplicates two authors (C.P., E.G.) independently screened the titles and abstracts of identified articles against the eligibility criteria. Cases of disagreement were resolved between the two reviewers; remaining uncertainties were resolved in consultation with C.M. Both reviewers then screened the full texts of the articles. Three authors (C.P., E.G., C.M.) determined the final inclusion.
Data extraction and analysis
A data extraction form was used that included bibliographic information (including country, setting, year of publication), study design and methods, participant characteristics, and main findings (Supplementary Table S2). Two authors (C.P., E.G.) independently completed the data extraction for each study, which was checked for accuracy and completeness by C.M.
A narrative synthesis was conducted, following the framework established by the Economic and Social Research Council (ESRC).4 The ESRC framework consists of (1) developing a theory or hypothesis, (2) preliminary synthesis of findings of included studies, (3) exploration of relationships within and between studies, and (4) assessment of the robustness of the synthesis. This approach aims to produce a textual, narrative understanding of findings and synthesize conceptual themes. Narrative synthesis was considered appropriate as we aimed to identify implementation factors across a broad body of evidence that included studies conducted in different settings and with quantitative, qualitative, and mixed designs. Two authors (C.P., E.G.) used tabulation and thematic analysis to extract and synthesize data from included studies, using the data extraction summaries and referring to the full text papers. Similarities and differences were then explored across the studies, key domains were identified, and patterns and relationships were grouped into themes. Themes identified by the synthesis were refined until consensus among all authors was reached.
Quality assessment
We assessed the quality of papers using a checklist that scores papers out of five5 (see Supplementary Table S3). We did not exclude papers with a lower quality score but used the scores to provide one indicator of the robustness of the synthesis.
RESULTS
Study characteristics
A total of 55 papers met the inclusion criteria (Supplementary Table S4). Most studies (n = 29) were from the United States, with the remaining from the UK (n = 10), Canada (n = 4), The Netherlands (n = 2), Estonia (n = 1), South Korea (n = 1), and Cuba (n = 1). One study was conducted across the United States and Canada. Six papers were systematic reviews. Studies focused on genetics and/or genomics, including genetic testing (targeted or panel tests), genetic counseling, genome and exome sequencing, and pharmacogenomics.
The studies were either interventional or observational. Interventional studies implemented and evaluated novel interventions into clinical practice. These included genetics/genomics education for clinicians, demonstrating the effectiveness or feasibility of care models, and testing digital tools. Observational studies examined aspects of clinical practice. These included perceptions and knowledge of genetics/genomics among clinicians and patients; analyzing routine data, for example referral rates to genetic services; and surveying health service organization. Both used a range of methods, including pre/post design, randomized controlled trials, surveys, observation, semistructured interviews, and statistical data analysis. Study participants were largely nongenetics health professionals, but also genetics counselors, patients, members of the public, and research participants.
Domains
The narrative synthesis of the literature identified four domains: (1) systems, (2) training and workforce, (3) professional attitudes and values, and (4) the role of patients and the public.
Systems
Service organization
Five papers described service organization, identifying inequity and variability in provision, and lack of coordination between genetics and nongenetics services.6,7,8,9,10 Two reviews of specialized services in the UK found variation in referral and genetic testing rates between regions,6 and an inverse relationship between referrals and deprivation.7
Variability was also reported in a survey of the US Veterans Health Administration (VHA) genetics services.8 Services were largely delivered through multidisciplinary clinics or coordinated services, but this was not consistent. For some clinicians, genetics services were only available at different clinical sites, via telemedicine, or at non-VHA facilities.
Clinicians in the United States and UK reported lack of service coordination as a barrier to integrating genetics services.9,10 In UK general practice, staff attitudes reflected institutional arrangements and commissioning decisions that regarded genetics as “specialist” and “peripheral” to mainstream services.10
Digital systems
Nine papers described the use of digital systems, evaluating clinical decision support (CDS) tools and investigating the ability of electronic health records (EHRs) to organize genetic information.11,12,13,14,15,16,17,18,19 The development of interoperable digital systems and data storage facilities has been identified as central to mainstreaming genomic medicine in the NHS.20 Studies we identified described challenges to implementing digital systems.
Six evaluated digital decision support.11,12,13,14,15,16 Digital reminders increased family history documentation and referral rates for patients in two studies.11,12 Two studies evaluated pharmacogenomics CDS.13,14 One found that clinicians considered the alerts helpful for prescribing decisions;13 the other reported that clinicians found alerts confusing and frustrating and had little impact on prescribing decisions.14 Barriers to implementing pharmacogenomics CDS were reported across 11 clinical sites, however these were found to be caused by general information technology (IT) problems, and not specific to genomic medicine.15 A systematic review of digital CDS for genetics concluded that further research is needed to understand how CDS can be integrated with current systems.16
Three studies described challenges when using EHRs to organize genetic or genomic data, suggesting that current systems were not ready to meet the future demands of genomic medicine.17,18,19 Few US-based clinicians (genetics and nongenetics specialists) and EHR representatives (including chief/executive medical officers, product managers, and IT specialists) felt EHRs met their current genomic medicine needs.17 Respondents stated the need for structured and standardized data elements such as functions to order genetic tests, results organized and displayed in pedigree format, and the ability to interpret familial risk. This finding was reflected in a US study of genetic test reporting in EHRs: no standard reporting format was used by the laboratories. EHRs were described as serving as storage for textual reports rather than meaningful structured data.18 Sperber et al.19 identified integrating genomics into EHRs as a challenge for US service providers, however using data warehousing techniques was found to aid integration across organizations.
Policies and guidelines
Seven papers described the impact of policies and guidelines on integrating genomics/genetics into practice.10,21,22,23,24,25,26
Clinicians reported a lack of guidelines for pharmacogenomics testing,21 the collection of family health history,22 and the disclosure of secondary findings.23 Lack of guidelines was cited by clinicians as a barrier to genetics service integration, but it could not be determined whether this finding resulted from an actual lack or lack of clinician awareness.24
Two studies described difficulties translating policies or guidelines into practice.25,26 In the UK, clinical genetics guidelines conceptualize genetic information as confidential to families rather than individuals. Despite this, UK-based clinicians reported that decision-making around confidentiality and disclosure remained based on an individual model.25 A US study examining the impact of an insurance-mandated requirement for genetic counseling prior to testing forBRCA1 and BRCA2 found that, contrary to the policy’s purpose, a higher number of people did not complete genetic testing after policy introduction.26
Changes to health service funding impacted on the delivery of genetic services in the UK, leading to the discontinuation of pilot genetics services in general practice10 and a low prevalence of follow-up appointments, preventing familial communication about genetic information.25 Responsibility for the governance and allocation of funding for UK genetic services was also reported as “ambiguous.”10
Access
Five studies described patient access to genetics services.27,28,29,30,31 Clinic or hospital location, along with patient ability to pay and health insurance coverage, were more frequently cited as barriers to genetic counseling by US-based genetics professionals (genetic counselors and genetics service providers) than patient attitudes, norms, and education.27,28 However, social factors, such as discouragement by family members, were also identified.28
Changing clinic location facilitated access in two studies.29,30 Delivering genetic counseling in primary care (general practice) compared with secondary care (hospital) led to higher rates of referral and attendance in a UK-based trial.29 In a US study, telemedicine enabled access to genetics services by saving patients’ time and travel costs.30
A systematic review of factors acting as barriers to patient referral to genetics services found that few studies focused on access. The evidence examined did not differentiate between access to referrals and the utilization of services.31
Health service costs
Four papers analyzed the organizational costs of genomic medicine.24,29,32,33
Two examined the cost-effectiveness of GS.32,33 A systematic review found that the current health economic evidence base to support use of genome and exome sequencing is limited and called for more studies evaluating costs and cost-effectiveness.32 A cost analysis of a randomized controlled trial delivering GS in US primary care found that short-term costs were driven primarily by the costs of sequencing, interpretation, and disclosure, but did not find evidence that GS increased downstream costs such as health-care utilization.33
Two papers investigated the costs of integrating genetics services.24,29 Clinicians perceived costs of unreimbursed time spent counseling and ordering tests as a barrier to integration.24 However, clinic costs (measured by staff travel and transportation) were not increased by delivering genetic counselor appointments in UK general practice compared with tertiary/secondary care.29
Training and workforce needs
Clinician preparedness
Twenty one papers describing the preparedness of clinicians9,14,21,22,23,24,28,31,34,35,36,37,38,39,40,41,42,43,44,45,46 found some variations across specialities, but overall clinicians lacked knowledge and/or confidence to implement genomic medicine into practice.9,21,22,34 Reports described little direct experience with using genetic services;22,35,36 feeling unprepared to order tests,21,37 and to interpret and disclose results9,22,38 and secondary findings;23 inexperience with using pharmacogenomics information;14 and feeling unprepared to respond to patient queries about direct-to-consumer testing.39
Clinicians’ lack of knowledge and awareness could act as a barrier to patients accessing and being referred to genetic services,31 such as genetic counseling.28,41 Shields et al.42 found reduced utilization of genetic tests and referrals among US clinicians who served populations with higher proportions of ethnic minority groups, but it was unclear whether the finding was due to clinician training or reflected the populations’ different needs.
A lack of comprehensive genetics/genomics training was identified in two surveys, of clinicians in Canada21 and medical course directors in the United States and Canada.40 Most clinicians reported that they had not received graduate or postgraduate training in pharmacogenomics or genetics.21 Course directors agreed that current medical training was insufficient preparation for using genetics/genomics in clinical practice.40
Differences were identified in the knowledge and skills across nongenetic specialities.9,21,35,38 A survey of US clinicians found that, depending on specialty, respondents had different expectations of the skills required. Areas such as neurology and oncology were expected to be more skilled in genetic risk assessment, testing, and management compared with cardiology and primary care.35
Limited experience with genetic and genomic information among the primary care workforce (family physicians and general practitioners) was highlighted in three studies.34,44,45 Study respondents expressed discomfort with discussing inheritance patterns; the contribution of genetics to common, complex disease; and communicating potential risk to family members.44 Lacking direct clinical experience, they reported that personal experiences, such as the experiences of family and friends, influenced their attitudes toward and perceptions of genomic medicine.34 This lack of knowledge was cited as a barrier to the integration of genetic services in two systematic reviews.24,43
However, primary care clinicians did report feeling comfortable talking to patients about basic genetics and taking a family history,44 and demonstrated an understanding of direct-to-consumer reports45 and the ability to manage and make appropriate clinical recommendations from GS results.46
Genomics/genetics education
Seven papers investigated educational interventions for clinicians, including six evaluations of novel education interventions11,29,47,48,49,50 and one systematic review.51 The studies reported that education interventions improved genetics knowledge among clinicians. One reported an increase in referral rates to genetics services.29 The systematic review found insufficient evidence to inform future educational interventions and recommended interventions should be assessed by changes in practice, such as patient management, rather than knowledge and confidence of clinicians.51
Genetics/genomics specialists
The role of genetics or genomics specialists was described in eight papers.8,22,24,30,31,34,52,53 Five8,22,24,34 reported that clinicians had variable or limited access to genetics services and reported having “unfamiliar” relationships with geneticists. The lack of genetics expertise across the workforce was identified as a barrier to patient referral to genetic services in a systematic review.31
Three described use of multidisciplinary teams (MDTs) to deliver specialized genetics services.30,52,53 Evaluation of a US pediatric telemedicine service demonstrated a positive impact on patients who reported high satisfaction, especially in underserved areas, because of the model’s flexibility and decrease in waiting times.30 Members of a rare disease MDT in the UK described how they valued the clinical and scientific diversity to make informed decisions about eligibility for GS, though it was acknowledged the MDT was resource intensive and, beyond certain conditions, remains unusual in UK health services.52 A pre/post study evaluating MDTs to treat inherited retinal dystrophy found that the model was delivered consistently but it was not clear what impact the MDT had on the overall outcomes of the study.53
Professional attitudes and values
Clinician attitudes
Nine papers investigated clinicians’ attitudes to genomic medicine, demonstrating that clinicians held positive beliefs about the potential of genomic medicine.9,22,23,35,36,37,38,44,54 However, several risks were identified, including misinterpretation of results by clinicians23 or patients,44 clinician errors in ordering genetic tests,54 and fear of causing patients unnecessary stress44 or harm.54 One study highlighted the potential risks of disclosing secondary findings, asserting that clinicians need to consider not only clinical utility but psychosocial, ethical, and legal factors.23
Five described clinician concerns about the clinical utility, defined as evidence of improving prediction, treatment, and management of disease and enabling clinical decision-making, and applicability of genomic medicine.9,22,35,37,54 Some concerns related specifically to mainstream use of GS.23,38 The added value of genetic testing, compared with existing practice, for predicting disease or treatment outcomes was also viewed as ambiguous by clinicians across five specialties in a US-based study.35
Compatibility with current practice and values
Fifteen papers highlighted the level of compatibility of genetics or genomic medicine to current practice and values.9,10,22,23,24,25,35,36,37,38,52,55,56,57,58 Four used diffusion of innovation theory59 to assess how certain attributes, including compatibility with organizational and individual values, norms, and needs, influenced adoption into routine use.22,35,36,58
In terms of practice, clinicians expressed concerns about the impact on workload and workflow,9,37 specifically a lack of time to order tests or explain results,24,36 and, for some clinicians, the complex logistics involved in ordering and receiving approval for genetic tests.35
Clinician views on compatibility varied according to type of test; overall, genetic testing was judged on clinical utility, in terms of whether use could inform clinical management and decision-making.35,58 This included consideration of the patient population and the condition. Genetic testing for colorectal cancer was perceived as of low need by clinicians working in one organization due to the older patient age served by the provider.22 In a US survey, most clinicians agreed that predictive testing for conditions where there is no available treatment, such as Huntington's disease, was compatible with professional and personal beliefs.36
Compatibility with professional role was perceived differently across specialties. Clinicians and staff working in general practice, family, or internal medicine did not feel delivering genetics services was part of their role, or were unclear about their role in providing genetics services,9,10,24 and tended to view genomic medicine as complex compared with those working in specialisms such as gynecology and pediatrics.36
Compatibility also included consideration of health organization/provider values. The US Veterans Health Administration viewed the mainstreaming of genetic services as incompatible with system values of low cost and high clinical impact.35 However, a study from Cuba found genetic services were adopted successfully into national health services, challenging the assumption that a personalized model of care is a prerequisite to the expansion and translation of genomics.55
Issues around confidentiality and secondary findings presented challenges to clinicians’ sense of responsibility toward patients in three UK studies.23,25,57 Clinicians described a struggle to both use a familial approach to confidentiality25 and act in the patients’ best interests, exacerbated by a lack of guidelines and evidence.23 Interviews with research participants found that decisions to disclose secondary findings need to consider individual patients’ tolerance for uncertainty.57 Research recruitment within health-care settings also posed challenges for UK health-care professionals. Recruitment targets for the 100,000 Genomes Project affected decision-making over eligibility and suitability for GS,57 and overshadowed commitments to patient informed consent.56
Patients and public
Patient and public involvement has been identified as key to the successful delivery of genomic medicine in the NHS.2 The knowledge, awareness, and engagement of patients and the public was described in nine papers.19,28,29,43,57,60,61,62,63 Studies demonstrated that patients and members of the public were aware of and generally held positive attitudes toward genetic testing61 but were less informed about the role of genes in disease62 and genetic services available to them.31,43 This low awareness could act as a barrier to referral to services such as genetic counseling.28
Patients and research participants often overestimated the potential of genetic testing or GS to provide clinical benefits.60,63 Engaging and educating patients was identified to address this.43,60,61,62,63 One study19 recommended specific strategies, including actively involving patients in implementation and decision-making.
Six papers described patient outcomes of genetic/genomic services.26,29,30,43,46,53 One systematic review reported that genetic/genomic services or interventions for common chronic diseases had modest positive effects on psychological outcomes and mixed behavioral outcomes.43 Three used patient-reported measures to assess psychological and behavioral outcomes of genetics/genomics services: a specialized service for ophthalmology patients,53 genetic counseling prior to BRCA1 and BRCA2 testing,26 and healthy patients receiving GS.46 All three studies demonstrated little significant change in patient-reported outcomes. Participants in the GS trial did report making health behavior changes related to the results and perceived them as medically useful in terms of influencing their medical treatment.46 However, some differences were found: the trial participants who received GS results expressed slightly lower levels of satisfaction and confidence with how well they understood the information compared with those who received a family history report only.
Two studies evaluated patient satisfaction.29,30 A US telemedicine pediatric clinic seeking to identify nonsyndromic developmental delay reported high satisfaction,30 while no difference in level of patient satisfaction was found when comparing genetic counseling (for cancer and noncancer conditions) delivered in general practice or a hospital setting.29
DISCUSSION
Genomic medicine is being mainstreamed into routine preventive, diagnostic, and interventional health care, bringing challenges for how health-care organizations may need to change to deliver this new technology. Our review identifies current knowledge about factors that will enable delivery of genomic medicine, focusing on four categories of interest: systems, training and workforce needs, professional attitudes and values, and the role of patients and the public.
The majority of studies reviewed were conducted in the United States. Differences in the governance and financing of US health services means that not all the domains identified will be relevant to the UK. In the US, patient ability to pay and health insurance coverage will factor into access to genetics/genomics services. Equally, prioritizing cost-effectiveness may limit NHS patient access to genetics and genomics services. US studies8,9,15,22,35,58 conducted in the VHA, along with findings from countries with some level of nationally funded health service—and the domains addressed in these studies—may be more immediately relevant to the UK setting.
However, health systems in these settings operate in contexts of varied economic, political, and social factors. This means that although genomic medicine seeks to transform global health systems, implementation will vary across and within local contexts. Here we draw out implications for the UK, acknowledging important differences that exist between health service structure and organization, to identify key issues shared across largely Western/industrialized contexts.
Through a systematic synthesis of current evidence on genomic medicine implementation across a range of domains, our review provides an overview of the key factors influencing health service readiness. The findings support existing recommendations63,64 that identify practical challenges at each level of clinical practice, including coordinated infrastructure and a trained and prepared workforce. We also found that the compatibility of professional, patient, and system values with genomic medicine plays a key role. This echoes the Generation Genome report, which states that genomic medicine is as much a “cultural and political exercise” as a scientific one.2 Summarizing our findings we have, thus, identified three overarching themes: reforming practice (referring to systems, training, and workforce); the value of genomic medicine (referring to professional attitudes and values); and revising the social contract (acknowledging the role of patients and the public).
Reforming practice
Our review suggests that at least some genetic services are not well integrated into clinical practice, raising questions over service coordination and equity of access. Acknowledging the parity of access and “cottage industry” of NHS genetic services, the Generation Genome report outlined intentions to streamline and centralize services, embedding national standards, in preparation for the Genomic Medicine Service. Papers we reviewed highlighted the need for guidelines to aid clinicians in tasks such as ordering genetic tests, making appropriate referrals, and interpreting results.
The need for standardized digital systems was identified, in particular consistent reporting formats and digital decision support tools in EHRs. Developing digital infrastructure in the NHS is a priority, specifically the capacity to store genetic data, linking local sites to a central database, and integrating genetic information into EHRs.20 Our review identified little literature that focused on the interoperability of systems or addressed informatics capabilities for managing GS data. However, findings from genomic research projects have proposed digital solutions and standards, which may in the future translate into clinical practice.19,65
Regarding training and workforce needs, in accordance with other systematic reviews, we identified differences in the knowledge and skills of those working in specialisms and those working in primary care (general practice or family medicine).23,42,66 This was reflected in the attitudes of staff in primary care settings, who regarded genetics as of little relevance to their practice. In current primary care practice, genetics and genomics may feature rarely, however the “whole NHS” approach proposed by the Genomic Medicine Service indicates that in the medium to long term the skills expected of the UK primary care workforce are likely to change.
Developing genetics/genomics education programs for nongenetic health professionals has therefore become a priority. The NHS aims to produce a “genomic literate” workforce, reforming training and education by integrating a new “genomic paradigm” into current medical curricula.2 The impact of these initiatives will become evident in the long term. Our review demonstrated education programs had mixed success and the long-term impact remains unclear. This finding indicates the limitations of isolated interventions; multifaceted health interventions have been found to be more likely to improve practice.67 A systematic review of genetics/genomics education for nongenetic health professionals also highlighted shortcomings in program design and evaluation.68,69 To date there has been a lack of engagement with implementation science frameworks in genomic medicine literature,3 yet our review identifies that successful education may require a wider reclarification of roles, norms, and values.
Along with training new and existing staff, developing a multidisciplinary approach to delivering genomic medicine has been prioritized.20 We found few descriptions of MDTs in practice. Prevalence varies between specialty, and refining our search to a specific condition, such as cancer, may have yielded more results.
The value of genomic medicine
Papers we reviewed emphasized the need for robust evidence of the clinical utility of genomic medicine, that is, evidence that treatments improve patient outcomes and/or enable clinical management. A 2008 review identified a lack of evidence for clinical outcomes, demonstrating the persistence of the problem.42 The lack of demonstrable clinical utility or benefit raises broader questions about the value of genomic medicine. Vassy et al.69 argued that the common understanding of clinical utility may need revising to account for genomic medicine. Considering instead the “appropriateness” of a treatment would involve evaluating whether the expected benefits exceed the expected negative consequences. For example, GS could be deemed appropriate if it serves to end the diagnostic odyssey and inform reproductive choices. For certain conditions, a diagnosis could also change clinical outcomes by enabling patients to receive treatment earlier, improving prognosis.
The appropriateness of genomic medicine may then include not only clinical utility but also the values of professionals and patients. Studies of patient and public attitudes toward genetic testing suggest they are driven by a broader understanding of utility that includes gaining a sense of control over one’s health70 or a moral imperative to undertake testing;71 similar sentiments may apply to genomics. Review findings indicate patients, the public, and research participants can overestimate the benefits of genetic testing and GS, suggesting a gap between the expectations and reality of what both can provide.
Clinical benefits may include changes in patient behavior, often cited as a rationale for genomic medicine. Papers we reviewed demonstrated only modest effects. The impact of genetic results on behavior has been shown to be limited.72,73 Christensen and Green,74 drawing on preliminary trial results, speculate that genomic information, specifically secondary findings from GS, could foster health behavior change unlike typical risk assessments.
Value refers not only to clinical benefits: genomic medicine may help to deliver care that is cost-effective, providing value for money spent.4 We found little evidence for cost-effectiveness or impact on health service costs, indicating the complexity of estimating and evaluating cost-effectiveness and economic benefit. Nevertheless, genomic medicine in the UK is positioned as an investment opportunity that, it is claimed, will boost the national economy by generating jobs and helping to develop a competitive research environment.
Rethinking the social contract
Mainstreaming genomic medicine, according to the Generation Genome report,4 signals a need to rethink the “social contract” set out in the NHS constitution that describes how patients, the public, and staff are bound together by shared principles and responsibilities. Rethinking the social contract would involve broadening patient consent and the current “narrow” model of confidentiality, balancing the interests of patients against those of family members and “broader society.”
As the “mainstreaming agenda” is implemented, questions about confidentiality and familial communication are likely to become more pertinent. Review findings suggest that expanding the traditional model of patient–doctor confidentiality may be difficult to implement in practice, and clinicians expressed concerns over patient harm. This difficulty may reflect a disjuncture between the focus on personalized care, of which genomics is considered a central part, and some of the implications of mainstreaming genomic medicine, which may force clinicians to reassess their understanding of responsibility.
The NHS vision states that clinicians’ duty of care toward patients (and family members) should be extended to researchers, bioinformaticians, and data managers. In genetics, it is well acknowledged that the boundaries between care and research are often blurred.75,76 Genomic medicine heightens issues around secondary findings and patient confidentiality, provoking further concerns around balancing informed consent and clinical benefits, and requiring better efforts to engage and inform the public about genomic data use.
Our review has some limitations. First, it is possible that despite using validated databases relevant articles that were not indexed and/or were written in languages other than English were not identified. Second, we used a broad search strategy to capture the range of factors involved in the implementation of genomic medicine. As such, our search returned studies on both genetics and genomics and we have sought to clarify the specificities between the two. Third, while the impact on nongenetic professionals remained our focus, studies involving geneticists/genetic counselors were included, judged on relevance to routine care. Fourth, although our synthesis focused on the requirements of the NHS to implement genomic medicine, our review draws on mostly non-UK literature and we have sought to address important differences between the health services of the included papers.
Despite these limitations, this systematic review contributes to the clinical genomics and genetics field by highlighting key actions required to implement genomic medicine into routine practice. In particular, our review has highlighted the new obligations and responsibilities that are being demanded of patients, clinicians, and health services, demonstrating not only the organizational, but also the social and cultural implications of reforming practice. Following on from the completion of the 100,000 Genomes Project, implementation of the NHS Genomic Medicine Service will likely accelerate. The UK provides an example for health services worldwide that seek to implement genomic technologies into routine practice. As such the results of this review may guide future integration of genomic medicine in the UK and globally.
Change history
25 June 2019
In sub-section ‘Genetics/genomics specialists’ sentence beginning ‘Five...’ cited reference 32 (Schwarze et al. 2018) and should have been reference 34 (Carroll et al. 2016). While in sub-section ‘The value of genomic medicine’ sentence beginning ‘V... .’ should have read ‘Vassy et al. ...’. Finally, in the same sub-section, sentence beginning ‘Christensen and,’ should have read ‘Christensen and Green’. The PDF and HTML versions of the Article have been modified accordingly.
References
Turnbull C, Scott RH, Thomas E, et al. The 100 000 Genomes Project: bringing whole genome sequencing to the NHS. BMJ. 2018;361:k1687.
Davies S. Generation Genome: annual report of the Chief Medical Officer. London: Department of Health; 2017.
Roberts MC, Kennedy AE, Chambers DA, Khoury MJ. The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. 2017;19:858–863.
Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews: a product of the ESRC methods programme (Version I). Lancaster, UK: University of Lancaster; 2006.
Dixon-Woods M, Cavers D, Agarwal S, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med Res Methodol. 2006;6:35.
Burton H, Alberg C, Stewart A. Mainstreaming genetics: a comparative review of clinical services for inherited cardiovascular conditions in the UK. Public Health Genomics. 2010;13:235–245.
McDonald K, Iredale R, Higgs G. The geography of genetics: an analysis of referral patterns to a cancer genetics service. Genomic Med. 2007;1:129–138.
Scheuner MT, Marshall N, Lanto A, et al. Delivery of clinical genetic consultative services in the Veterans Health Administration. Genet Med. 2014;16:609–619.
Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Pers Med. 2010;7:485–494.
Martin G, Currie G, Finn R. Bringing genetics into primary care: findings from a national evaluation of pilots in England. J Health Serv Res Policy. 2009;14:204–211.
Scheuner MT, Hamilton AB, Peredo J, et al. A cancer genetics toolkit improves access to genetic services through documentation and use of the family history by primary-care clinicians. Genet Med. 2014;16:60–69.
Buchanan AH, Christianson C, Himmel T, et al. Use of a patient-entered family health history tool with decision support in primary care: impact of identification of increased risk patients on genetic counseling attendance. J Genet Couns. 2015;24:179–188.
Devine EB, Lee C-J, Overby CL, et al. Usability evaluation of pharmacogenomics clinical decision support aids and clinical knowledge resources in a computerized provider order entry system: a mixed methods approach. Int J Med Inform. 2014;83:473–483.
St Sauver JL, Bielinski SJ, Olson JE, et al. Integrating pharmacogenomics into clinical practice: promise vs reality. Am J Med. 2016;129:1093–1099.
Herr TM, Bielinski SJ, Bottinger E, et al. Practical considerations in genomic decision support: the eMERGE experience. J Pathol Inform. 2015;6:50.
Welch BM, Kawamoto K. Clinical decision support for genetically guided personalized medicine: a systematic review. J Am Med Inform Assoc. 2013;20:388–400.
Scheuner MT, de Vries H, Kim B, Meili RC, Olmstead SH, Teleki S. Are electronic health records ready for genomic medicine? Genet Med. 2009;11:510–517.
Ronquillo JG, Li C, Lester WT. Genetic testing behavior and reporting patterns in electronic medical records for physicians trained in a primary care specialty or subspecialty. J Am Med Inform Assoc. 2012;19:570–574.
Sperber NR, Carpenter JS, Cavallari LH, et al. Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med Genomics. 2017;10:35.
House of Commons Science and Technology Committee. Genomics and genome editing in the NHS. 2018. https://publications.parliament.uk/pa/cm201719/cmselect/cmsctech/349/349.pdf. accessed 06 Jun 2019.
Amara N, Blouin-Bougie J, Bouthillier D, Simard J. On the readiness of physicians for pharmacogenomics testing: an empirical assessment. Pharmacogenomics J. 2017;18:308–318.
Sperber NR, Andrews SM, Voils CI, Green GL, Provenzale D, Knight S. Barriers and facilitators to adoption of genomic services for colorectal care within the Veterans Health Administration. J Pers Med. 2016;6:E16.
Ormondroyd E, Mackley MP, Blair E, et al. “Not pathogenic until proven otherwise”: perspectives of UK clinical genomics professionals toward secondary findings in context of a Genomic Medicine Multidisciplinary Team and the 100,000 Genomes Project. Genet Med. 2018;20:320–328.
Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers’ perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17:169–176.
Dheensa S, Fenwick A, Lucassen A. Approaching confidentiality at a familial level in genomic medicine: a focus group study with healthcare professionals. BMJ Open. 2017;7:e012443.
Stenehjem DD, Au T, Sainski AM, et al. Impact of a genetic counseling requirement prior to genetic testing. BMC Health Serv Res. 2018;18:165.
Markens S. “I’m not sure if they speak to everyone about this option”: analyzing disparate access to and use of genetic health services in the US from the perspective of genetic counselors. Crit Public Heath. 2017;27:111–124.
Rolnick SJ, Rahm AK, Jackson JM, et al. Barriers in identification and referral to genetic counseling for familial cancer risk: the perspective of genetic service providers. J Genet Couns. 2011;20:314–322.
Westwood G, Pickering R, Latter S, et al. A primary care specialist genetics service: a cluster-randomised factorial trial. Br J Gen Pract. 2012;62:e191–7.
Kubendran S, Sivamurthy S, Schaefer GB. A novel approach in pediatric telegenetic services: geneticist, pediatrician and genetic counselor team. Genet Med. 2017;19:1260–1267.
Delikurt T, Williamson GR, Anastasiadou V, Skirton H. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet. 2015;23:739–745.
Schwarze K, Buchanan J, Taylor JC, Wordsworth S. Are whole-exome and whole-genome sequencing approaches cost-effective? A systematic review of the literature. Genet Med. 2018;20:1122–1130.
Christensen KD, Vassy JL, Phillips KA, et al. Short-term costs of integrating whole-genome sequencing into primary care and cardiology settings: a pilot randomized trial. Genet Med. 2018;20:1544–1553.
Carroll JC, Makuwaza T, Manca DP, et al. Primary care providers’ experiences with and perceptions of personalized genomic medicine. Can Fam Phys. 2016;62:E626–E635.
Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16:238–245.
Suther SG, Goodson P. Texas physicians’ perceptions of genomic medicine as an innovation. Clin Genet. 2004;65:368–377.
Chase DA, Baron S, Ash JS. Clinical decision support and primary care acceptance of genomic medicine. Stud Health Technol Inform. 2017;245:700–703.
Christensen KD, Vassy JL, Jamal L, et al. Are physicians prepared for whole genome sequencing? A qualitative analysis. Clin Genet. 2016;89:228–234.
Powell KP, Christianson CA, Cogswell WA, et al. Educational needs of primary care physicians regarding direct-to-consumer genetic testing. J Genet Couns. 2012;21:469–478.
Plunkett-Rondeau J, Hyland K, Dasgupta S. Training future physicians in the era of genomic medicine: trends in undergraduate medical genetics education. Genet Med. 2015;17:927–934.
Leach E, Morris E, White HJ, Inglis A, Lehman A, Austin J. How do physicians decide to refer their patients for psychiatric genetic counseling? A qualitative study of physicians’ practice. J Genet Couns. 2016;25:1235–1242.
Shields AE, Burke W, Levy DE. Differential use of available genetic tests among primary care physicians in the United States: results of a national survey. Genet Med. 2008;10:404–414.
Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. JAMA. 2008;299:1320–1334.
Leitsalu L, Hercher L, Metspalu A. Giving and withholding of information following genomic screening: challenges identified in a study of primary care physicians in Estonia. J Genet Couns. 2012;21:591–604.
Bernhardt BA, Zayac C, Gordon ES, Wawak L, Pyeritz RE, Gollust SE. Incorporating direct-to-consumer genomic information into patient care: attitudes and experiences of primary care physicians. Pers Med. 2012;9:683–692.
Vassy JL, Christensen KD, Schonman EF, et al. The impact of whole-genome sequencing on the primary care and outcomes of healthy adult patients: a pilot randomized trial. Ann Intern Med. 2017;167:159.
Clyman JC, Nazir F, Tarolli S, Black E, Lombardi RQ, Higgins JJ. The impact of a genetics education program on physicians’ knowledge and genetic counseling referral patterns. Med Teach. 2007;29:E143–E150.
Houwink EJF, Muijtjens AMM, van Teeffelen SR, et al. Effect of comprehensive oncogenetics training interventions for general practitioners, evaluated at multiple performance levels. PLoS ONE. 2015;10:e0122648.
Carroll JC, Grad R, Allanson JE, et al. The Gene Messenger Impact Project: an innovative genetics continuing education strategy for primary care providers. J Contin Educ Health Prof. 2016;36:171–178.
Houwink EJF, Muijtjens AMM, van Teeffelen SR, et al. Effectiveness of oncogenetics training on general practitioners’ consultation skills: a randomized controlled trial. Genet Med. 2014;16:45–52.
Paneque M, Turchetti D, Jackson L, Lunt P, Houwink E, Skirton H. A systematic review of interventions to provide genetics education for primary care. BMC Fam Pract. 2016;17:89.
Ormondroyd E, Mackley MP, Blair E, et al. Insights from early experience of a Rare Disease Genomic Medicine Multidisciplinary Team: a qualitative study. Eur J Hum Genet. 2017;25:680–686.
Davison N, Payne K, Eden M, et al. Exploring the feasibility of delivering standardized genomic care using ophthalmology as an example. Genet Med. 2017;19:1032–1039.
Korngiebel DM, Fullerton SM, Burke W. Patient safety in genomic medicine: an exploratory study. Genet Med. 2016;18:1136–1142.
Gibbon SE. Science, sentiment, and the state: community genetics and pursuit of public health in Cuba. Med Anthropol Q. 2013;27:531–549.
Samuel GN, Farsides B. Genomics England’s implementation of its public engagement strategy: blurred boundaries between engagement for the United Kingdom’s 100,000 Genomes Project and the need for public support. Public Underst Sci. 2018;27:352–364.
Mackley MP, Blair E, Parker M, Taylor JC, Watkins H, Ormondroyd E. Views of rare disease participants in a UK whole-genome sequencing study towards secondary findings: a qualitative study. Eur J Hum Genet. 2018;26:652–659.
Lerner B, Marshall N, Oishi S, et al. The value of genetic testing: beyond clinical utility. Genet Med. 2017;19:763–771.
Rogers EM. Diffusion of innovations. 5th ed. New York: Free Press; 2003.
Eum H, Lee M, Yoon J, et al. Differences in attitudes toward genetic testing among the public, patients, and health-care professionals in Korea. Eur J Hum Genet. 2018;26:1432–1440.
Christianson CA, Powell KP, Hahn SE, et al. Findings from a community education needs assessment to facilitate the integration of genomic medicine into primary care. Genet Med. 2010;12:587–593.
Roberts JS, Robinson JO, Diamond PM, et al. Patient understanding of, satisfaction with, and perceived utility of whole-genome sequencing: findings from the MedSeq Project. Genet Med. 2018;20:1069–1076.
Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267.
Bowdin S, Gilbert A, Bedoukian E, et al. Recommendations for the integration of genomics into clinical practice. Genet Med. 2016;18:1075–1084.
Lochmüller H, Badowska DM, Thompson R, et al. RD-Connect, NeurOmics and EURenOmics: collaborative European initiative for rare diseases. Eur Hum Genet. 2018;26:778.
Suther S, Goodson P. Barriers to the provision of genetic services by primary care physicians: a systematic review of the literature. Genet Med. 2003;5:70–76.
Boaz A, Baeza J, Fraser A, the European Implementation Score Collaborative Group (EIS). Effective implementation of research into practice: an overview of systematic reviews of the health literature. BMC Res Notes. 2011;4:212.
Talwar D, Tseng T-S, Foster M, Xu L, Chen L-S. Genetics/genomics education for nongenetic health professionals: a systematic literature review. Genet Med. 2017;19:725–732.
Vassy JL, Bates DW, Murray MF. Appropriateness: a key to enabling the use of genomics in clinical practice? Am J Med. 2016;129:551–553.
Etchegary H. Public attitudes toward genetic risk testing and its role in healthcare. Pers Med. 2014;11:509–522.
Cowley L. What can we learn from patients’ ethical thinking about the right ‘not to know’ in genomics? Lessons from cancer genetic testing for genetic counselling. Bioethics. 2016;30:628–635.
Henrikson NB, Bowen D, Burke W. Does genomic risk information motivate people to change their behavior? Genome Med. 2009;1:37.
Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102.
Christensen KD, Green RC. How could disclosing incidental information from whole-genome sequencing affect patient behavior? Pers Med. 2013;10:377–386.
Hallowell N, Cooke S, Crawford G, Parker M, Lucassen A. Healthcare professionals’ and researchers’ understanding of cancer genetics activities: a qualitative interview study. J Med Ethics. 2009;35:113–119.
Hallowell N, Cooke S, Crawford G, Lucassen A, Parker M. Distinguishing research from clinical care in cancer genetics: theoretical justifications and practical strategies. Soc Sci Med. 2009;68:2010–2017.
Acknowledgements
We acknowledge the support of the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and the NIHR Collaboration for Leadership in Applied Health Research and Care South London at King’s College Hospital NHS Foundation Trust (NIHR CLAHRC-2013-10022). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pearce, C., Goettke, E., Hallowell, N. et al. Delivering genomic medicine in the United Kingdom National Health Service: a systematic review and narrative synthesis. Genet Med 21, 2667–2675 (2019). https://doi.org/10.1038/s41436-019-0579-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41436-019-0579-x
Keywords
This article is cited by
-
QALYs and rare diseases: exploring the responsiveness of SF-6D, EQ-5D-5L and AQoL-8D following genomic testing for childhood and adult-onset rare genetic conditions in Australia
Health and Quality of Life Outcomes (2023)
-
Uptake of funded genomic testing for syndromic and non-syndromic intellectual disability in Australia
European Journal of Human Genetics (2023)
-
The implementation of large-scale genomic screening or diagnostic programmes: A rapid evidence review
European Journal of Human Genetics (2023)
-
Patients’ and professionals’ views related to ethical issues in precision medicine: a mixed research synthesis
BMC Medical Ethics (2021)
-
Anticipating the primary care role in genomic medicine: expectations of genetics health professionals
Journal of Community Genetics (2021)