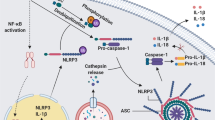
Abstract
Inflammasomes are key regulators of the host response against microbial pathogens, in addition to limiting aberrant responses to sterile insults, as mediated by environmental agents such as toxins or nanoparticles, and also by endogenous danger signals such as monosodium urate, ATP and amyloid-β. To date at least six different inflammasome signalling platforms have been reported (Bauernfeind & Hornung, EMBO Mol Med. 2013;5:814–26; Broz & Dixit, Nat Rev Immunol. 2016;16:407). This review focuses on the complex molecular machinery involved in activation and regulation of the best characterised inflammasome, NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3), and the development of molecular agents to modulate NLRP3 inflammasome function. Activation of the NLRP3 inflammasome induces inflammation via secretion of interleukin-1β (IL-1β) and interleukin-18 (IL-18) proinflammatory cytokines, with orchestration of pyroptotic cell death, to eliminate invading microbial pathogens. This field has gradually moved from an emphasis on monogenic autoinflammatory conditions, such as cryopyrin-associated periodic syndromes (CAPS), to the broad spectrum of innate immune-mediated disease. NLRP3 inflammasome activation is also linked to a range of common disorders in humans including type 2 diabetes (Krainer et al., J Autoimmun. 2020:102421), cystic fibrosis (Scambler et al., eLife. 2019;8), myocardial infarction, Parkinson’s disease, Alzheimer’s disease (Savic et al., Nat Rev Rheumatol. 2020:1–16) and cancers such as mesotheliomas and gliomas (Moossavi et al., Mol Cancer. 2018;17:158). We describe how laboratory-based assessment of NLRP3 inflammasome activation is emerging as an integral part of the clinical evaluation and treatment of a range of undifferentiated systemic autoinflammatory disorders (uSAID) (Harrison et al., JCI Insight. 2016;1), where a DNA-based diagnosis has not been possible. In addition, this review summarises the current literature on physiological inhibitors and features various pharmacological approaches that are currently being developed, with potential for clinical translation in autoinflammatory and immune-mediated conditions. We discuss the possibilities of rational drug design, based on detailed structural analyses, and some of the challenges in transferring exciting preliminary results from trials of small-molecule inhibitors of the NLRP3 inflammasome, in animal models of disease, to the clinical situation in human pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vande Walle L, Kanneganti T-D, Lamkanfi M. HMGB1 release by inflammasomes. Virulence. 2011;2:162–5.
Savic S, Caseley EA, McDermott MF. Moving towards a systems-based classification of innate immune-mediated diseases. Nat Rev Rheumatol. 2020;16:1–16.
Krainer J, Siebenhandl S, Weinhäusel A. Systemic autoinflammatory diseases. J Autoimmun. 2020;109:102421.
Zahid A, Li B, Kombe JK, Jin T, Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol. 2019;10:2538.
Hoffman HM. Therapy of autoinflammatory syndromes. J Allergy Clin Immunol. 2009;124:1129–38.
Wekell P, Berg S, Karlsson A, Fasth A. Toward an inclusive, congruent, and precise definition of autoinflammatory diseases. Front Immunol. 2017;8:497.
Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126:1260–80.
Romberg N, Vogel TP, Canna SW. NLRC4 inflammasomopathies. Curr Opin Allergy Clin Immunol. 2017;17:398.
Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248.
He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9:1–12.
Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214:3219–38.
Sarrauste de Menthière C, Terriere S, Pugnere D, Ruiz M, Demaille J, Touitou I. INFEVERS: the registry for FMF and hereditary inflammatory disorders mutations. Nucleic Acids Res. 2003;31:282–5.
Walle LV, Stowe IB, Šácha P, Lee BL, Demon D, Fossoul A, et al. MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition. PLoS Biol. 2019;17:e3000354.
Nakanishi H, Prakash P, Ito T, Kim HJ, Brewer CC, Harrow D, et al. Genetic hearing loss associated with autoinflammation. Front Neurol. 2020;11:141.
de Jesus AA, Hou Y, Brooks S, Malle L, Biancotto A, Huang Y, et al. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J Clin. Invest. 2020;130:1669–82.
Poulter JA, McDermott MA. Faculty Opinions Recommendation of [de Jesus AA et al., J Clin Invest 2020 130:1669–1682]. In Faculty Opinions. https://doi.org/10.3410/f.737127558.793571862.
Gram H. The long and winding road in pharmaceutical development of canakinumab from rare genetic autoinflammatory syndromes to myocardial infarction and cancer. Pharmacol Res. 2019;154:104139.
Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol 2018;9:1157.
Coburn LA, Horst SN, Chaturvedi R, Brown CT, Allaman MM, Scull BP, et al. High-throughput multi-analyte Luminex profiling implicates eotaxin-1 in ulcerative colitis. PloS ONE. 2013;8:e82300.
Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflamm. 2017;2017:4309485.
Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. 2012;189:4175–81.
Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454.
Mortimer L, Moreau F, MacDonald JA, Chadee K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat Immunol. 2016;17:1176.
Indramohan M, Stehlik C, Dorfleutner A. COPs and POPs patrol inflammasome activation. J Mol Biol. 2018;430:153–73.
Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–8.
Hu Y, Mao K, Zeng Y, Chen S, Tao Z, Yang C, et al. Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. J Immunol. 2010;185:7699–705.
Jin J, Yu Q, Han C, Hu X, Xu S, Wang Q, et al. LRRFIP2 negatively regulates NLRP3 inflammasome activation in macrophages by promoting Flightless-I-mediated caspase-1 inhibition. Nat Commun. 2013;4:1–8.
Carpentier SJ, Ni M, Duggan JM, James RG, Cookson BT, Hamerman JA. The signaling adaptor BCAP inhibits NLRP3 and NLRC4 inflammasome activation in macrophages through interactions with Flightless-1. Sci Signal. 2019;12:eaau0615.
Kubota T, Koike R. Cryopyrin-associated periodic syndromes: background and therapeutics. Mod Rheumatol. 2010;20:213–21.
Shim D-W, Lee K-H. Posttranslational regulation of the NLR family pyrin domain-containing 3 inflammasome. Front Immunol. 2018;9:1054.
Swanson KV, Deng M, Ting JP-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:1–11.
Song N, Li T. Regulation of NLRP3 inflammasome by phosphorylation. Front Immunol. 2018;9:2305.
Sokolowska M, Chen L-Y, Liu Y, Martinez-Anton A, Qi H-Y, Logun C, et al. Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. J Immunol. 2015;194:5472–87.
Zhang Z, Meszaros G, He W-t, Xu Y, de Fatima Magliarelli H, Mailly L, et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J Exp Med. 2017;214:2671–93.
Liu X, Pichulik T, Wolz O-O, Dang T-M, Stutz A, Dillen C, et al. Human NACHT, LRR, and PYD domain–containing protein 3 (NLRP3) inflammasome activity is regulated by and potentially targetable through Bruton tyrosine kinase. J Allergy Clin Immunol. 2017;140:1054–67.e10.
Mao L, Kitani A, Hiejima E, Montgomery-Recht K, Zhou W, Fuss I, et al. Bruton tyrosine kinase deficiency augments NLRP3 inflammasome activation and causes IL-1β-mediated colitis. J Clin Invest. 2020;130:1793–1807.
Bittner ZA, Liu X, Dickhoefer S, Kalbacher H, Bosch K, Andreeva L, et al. BTK operates a phospho-tyrosine switch to regulate NLRP3 inflammasome activity. bioRxiv. 2019. https://www.biorxiv.org/content/10.1101/864702v1.
Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–43.
Han S, Lear TB, Jerome JA, Rajbhandari S, Snavely CA, Gulick DL, et al. Lipopolysaccharide primes the NALP3 inflammasome by inhibiting its ubiquitination and degradation mediated by the SCFFBXL2 E3 ligase. J Biol Chem. 2015;290:18124–33.
Barry R, John SW, Liccardi G, Tenev T, Jaco I, Chen C-H, et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat Commun. 2018;9:1–14.
Paramel G, Sirsjö A, Fransén K. Role of genetic alterations in the NLRP3 and CARD8 genes in health and disease. Mediat Inflamm. 2015;2015:846782.
Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25.
Ito S, Hara Y, Kubota T. CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction. Arthritis Res Ther. 2014;16:R52.
Cheung MS, Theodoropoulou K, Lugrin J, Martinon F, Busso N, Hofer M. Periodic fever with aphthous stomatitis, pharyngitis, and cervical adenitis syndrome is associated with a CARD8 variant unable to bind the NLRP3 inflammasome. J Immunol. 2017;198:2063–9.
Mao L, Kitani A, Similuk M, Oler AJ, Albenberg L, Kelsen J, et al. Loss-of-function CARD8 mutation causes NLRP3 inflammasome activation and Crohn’s disease. J Clin Invest. 2018;128:1793–806.
Haq T, Richards MW, Burgess SG, Gallego P, Yeoh S, O’Regan L, et al. Mechanistic basis of Nek7 activation through Nek9 binding and induced dimerization. Nat Commun. 2015;6:1–12.
He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–7.
Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2015;17:250–8.
Chen X, Liu G, Yuan Y, Wu G, Wang S, Yuan L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 2019;10:1–12.
Matsubayashi T, Sugiura H, Arai T, Oh‐Ishi T, Inamo Y. Anakinra therapy for CINCA syndrome with a novel mutation in exon 4 of the CIAS1 gene. Acta Paediatr. 2006;95:246–9.
Jesus AA, Silva CA, Segundo GR, Aksentijevich I, Fujihira E, Watanabe M, et al. Phenotype–genotype analysis of cryopyrin-associated periodic syndromes (CAPS): description of a rare non-exon 3 and a novel CIAS1 missense mutation. J Clin Immunol. 2008;28:134.
Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–68.
Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha‐and beta‐subunits of ATP synthase, myosin, kinases and other ATP‐requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–51.
Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. PNAS. 2007;104:8041–6.
Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15:556–9.
Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal‐onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin‐associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–8.
Caroli F, Pontillo A, D’Osualdo A, Travan L, Ceccherini I, Crovella S, et al. Clinical and genetic characterization of Italian patients affected by CINCA syndrome. Rheumatology. 2007;46:473–8.
Zhang L, Chen S, Ruan J, Wu J, Tong AB, Yin Q, et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 2015;350:404–9.
Hu Z, Yan C, Liu P, Huang Z, Ma R, Zhang C, et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science. 2013;341:172–5.
Matyszewski M, Zheng W, Lueck J, Antiochos B, Egelman EH, Sohn J. Cryo-EM structure of the NLRC4CARD filament provides insights into how symmetric and asymmetric supramolecular structures drive inflammasome assembly. J Biol Chem. 2018;293:20240–8.
Oroz J, Barrera-Vilarmau S, Alfonso C, Rivas G, de Alba E. ASC pyrin domain self-associates and binds NLRP3 protein using equivalent binding interfaces. J Biol Chem. 2016;291:19487–501.
Vajjhala PR, Mirams RE, Hill JM. Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J Biol Chem. 2012;287:41732–43.
Bae JY, Park HH. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem. 2011;286:39528–36.
Abais JM, Xia M, Zhang Y, Boini KM, Li P-L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox. Sign. 2015;22:1111–29.
Hoffman HM, Scott P, Mueller JL, Misaghi A, Stevens S, Yancopoulos GD, et al. Role of the leucine‐rich repeat domain of cryopyrin/NALP3 in monosodium urate crystal–induced inflammation in mice. Arthritis Rheum. 2010;62:2170–9.
Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33.
Py BF, Kim M-S, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–8.
Hoss F, Mueller JL, Ringeling FR, Rodriguez-Alcazar JF, Brinkschulte R, Seifert G, et al. Alternative splicing regulates stochastic NLRP3 activity. Nat Commun. 2019;10:1–13.
Dowds TA, Masumoto J, Zhu L, Inohara N, Núñez G. Cryopyrin-induced interleukin 1β secretion in monocytic cells enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004;279:21924–8.
Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–34.
Hafner-Bratkovič I, Sušjan P, Lainšček D, Tapia-Abellán A, Cerović K, Kadunc L, et al. NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nat Commun. 2018;9:1–18.
Koda A, Nagai H, Watanabe S, Yanagihara Y, Sakamoto K. Inhibition of hypersensitivity reactions by a new drug, N (3′, 4′-dimethoxycinnamoyl) anthranilic acid (N-5′). J Allergy Clin Immunol. 1976;57:396–407.
Huang Y, Jiang H, Chen Y, Wang X, Yang Y, Tao J, et al. Tranilast directly targets NLRP3 to treat inflammasome‐driven diseases. EMBO Mol Med. 2018;10:e8689.
He Y, Varadarajan S, Muñoz-Planillo R, Burberry A, Nakamura Y, Núñez G. 3, 4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014;289:1142–50.
Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu J-W, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285:9792–802.
Shim D-W, Shin W-Y, Yu S-H, Kim B-H, Ye S-K, Koppula S, et al. BOT-4-one attenuates NLRP3 inflammasome activation: NLRP3 alkylation leading to the regulation of its ATPase activity and ubiquitination. Sci Rep. 2017;7:1–12.
Perregaux DG, McNiff P, Laliberte R, Hawryluk N, Peurano H, Stam E, et al. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J Pharm Exp Ther. 2001;299:187–97.
Tapia-Abellán A, Angosto-Bazarra D, Martínez-Banaclocha H, de Torre-Minguela C, Cerón-Carrasco JP, Pérez-Sánchez H, et al. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat Chem Biol. 2019;15:560–4.
Śledź P, Caflisch A. Protein structure-based drug design: from docking to molecular dynamics. Curr Opin Struc Biol 2018;48:93–102.
Abdullaha M, Mohammed S, Ali M, Kumar A, Vishwakarma RA, Bharate SB. Discovery of quinazolin-4 (3 H)-ones as NLRP3 inflammasome inhibitors: computational design, metal-free synthesis, and in vitro biological evaluation. J Org Chem. 2019;84:5129–40.
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–206.
Pal A, Neo K, Rajamani L, Ferrer FJ, Lane DP, Verma CS, et al. Inhibition of NLRP3 inflammasome activation by cell-permeable stapled peptides. Sci Rep. 2019;9:1–15.
Chang W-C, Chu M-T, Hsu C-Y, Wu Y-JJ, Lee J-Y, Chen T-J, et al. Rhein, an Anthraquinone drug, suppresses the NLRP3 Inflammasome and macrophage activation in urate crystal-induced gouty inflammation. Am J Chin Med. 2019;47:135–51.
Wally V, Hovnanian A, Ly J, Buckova H, Brunner V, Lettner T, et al. Diacerein orphan drug development for epidermolysis bullosa simplex: a phase 2/3 randomized, placebo-controlled, double-blind clinical trial. J Am Acad Dermatol. 2018;78:892–901.e7.
Marchetti C, Swartzwelter B, Gamboni F, Neff CP, Richter K, Azam T, et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. PNAS 2018;115:E1530–9.
Jansen T, Klück V, Janssen M, Comarniceanu A, Efdé M, Scribner C, et al. P160 The first phase 2A proof-of-concept study of a selective NLRP3 inflammasome inhibitor, dapansutrile™(OLT1177™), in acute gout. Ann Rheum Dis. BMJ Publishing Group Ltd. 2019; A70–1.
Inzomelid completes Phase I studies and shows positive results in the treatment of Cryopyrin-Associated Periodic Syndrome (CAPS). 2020.
Darakhshan S, Pour AB. Tranilast: a review of its therapeutic applications. Pharm Res. 2015;91:15–28.
Mangan MS, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588.
Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Castéran N, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PloS ONE. 2009;4:e7258.
Cikovic T. Effects of small molecules and cytokine signalling on NLRP3 inflammasome activation. Doctoral dissertation, Technische Universität München; 2019.
Mora JS, Genge A, Chio A, Estol CJ, Chaverri D, Hernández M, et al. Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomized clinical trial. Amyotroph Lateral Scler Frontotemporal Degeneration. 2020;21:5–14.
Science A. Masitinib significantly delays disability progression on EDSS in patients with primary progressive (PPMS) and non-active secondary progressive (nSPMS) multiple sclerosis. 2020. https://multiplesclerosisnewstoday.com/news-posts/2020/02/21/ab-science-announces-positive-top-line-phase-2b3-results-for-oral-masitinib-in-progressive-forms-of-multiple-sclerosis/.
Science A. Interim results of masitinib study in Alzheimer’s disease: positive trend of efficacy in one of the doses tested. 2019. http://www.ab-sciencecom/images/_pdf/CP_AD_Interim_VEng_VF.pdf.
Maier NK, Crown D, Liu J, Leppla SH, Moayeri M. Arsenic trioxide and other arsenical compounds inhibit the NLRP1, NLRP3, and NAIP5/NLRC4 inflammasomes. J Immunol. 2014;192:763–70.
Paugh SW, Bonten EJ, Savic D, Ramsey LB, Thierfelder WE, Gurung P, et al. The NLRP3-CASP1 Inflammasome Induces Glucocorticoid Resistance in ALL. Cancer Discov. 2015;5:18.
Pang Z, Wang G, Ran N, Lin H, Wang Z, Guan X, et al. Inhibitory effect of methotrexate on rheumatoid arthritis inflammation and comprehensive metabolomics analysis using ultra-performance liquid chromatography-quadrupole time of flight-mass spectrometry (UPLC-Q/TOF-MS). Int J Mol Sci. 2018;19:2894.
Tang T-T, Lv L-L, Pan M-M, Wen Y, Wang B, Li Z-L, et al. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018;9:1–14.
Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–23.
Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286:38703–13.
Acknowledgements
The authors (EC, JP and MMcD) are supported by the EU Horizon 2020 research and innovation program (ImmunAID; grant agreement number 779295); FR is supported the Foundation for Development of Internal Medicine in Europe (FDIME), the European Federation of Internal Medicine (EFIM) and the French National Society for Internal Medicine (SNFMI).
Immunome Project Consortium for Autoinflammatory Disorders (ImmunAID)
Emily A. Caseley1, James A. Poulter1, Michael F. McDermott1, Vassili Soumelis1
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Immunome project consortium for Autoinflammatory Disorders (ImmunAID) are listed below Acknowledgements.
Rights and permissions
About this article
Cite this article
Caseley, E.A., Poulter, J.A., Rodrigues, F. et al. Inflammasome inhibition under physiological and pharmacological conditions. Genes Immun 21, 211–223 (2020). https://doi.org/10.1038/s41435-020-0104-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-020-0104-x
This article is cited by
-
Muscimol inhibits plasma membrane rupture and ninjurin-1(NINJ1) oligomerization during pyroptosis
Communications Biology (2023)
-
Insights into inflammasome regulation: cellular, molecular, and pathogenic control of inflammasome activation
Immunologic Research (2022)
-
Inflammasomes and the IL-1 Family in Bone Homeostasis and Disease
Current Osteoporosis Reports (2022)
-
An Atypical Autoinflammatory Disease Due to an LRR Domain NLRP3 Mutation Enhancing Binding to NEK7
Journal of Clinical Immunology (2022)
-
Parenchymal neuroinflammatory signaling and dural neurogenic inflammation in migraine
The Journal of Headache and Pain (2021)