Abstract
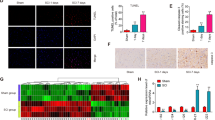
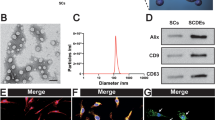
In this study, we aimed to investigate the therapeutic effect of miR-21 in the treatment of spinal cord injury (SCI) as well as its underlying molecular mechanisms. Real-time PCR and western blot were performed to measure the expression of miR-21, PTEN, and PDCD4 in SCI rats. Locomotion recovery assessment, Nissl staining, IHC assay, and TUNEL assay were utilized to observe the therapeutic effect of miR-21 in the treatment of SCI. Bioinformatics analysis and luciferase assay were conducted to establish the signaling pathway of miR-21, PTEN, and PDCD4. The regulatory relationships between miR-21 and PTEN/PDCD4 were further validated by real-time PCR, western blot, MTT assay, and flow cytometry. Compared with sham-operated rats, SCI rats showed decreased expression of miR-21 along with increased expression of PTEN/PDCD4. Exosomes were equally distributed in MSCs transfected with miR-21, PTEN siRNA, or scramble controls. The exosomes isolated from the supernatant of cultured MSCs could improve the functional recovery of SCI rats by reducing SCI-induced neuron loss. In addition, miR-21 was shown to inhibit the expression of PTEN/PDCD4 and suppress neuron cell death. Moreover, PTEN and PDCD4 were validated as virtual targets of miR-21. In addition, the miR-21/PTEN/PDCD4 signaling pathway was shown to enhance cell viability and suppress cell death in vivo. The exosomes collected from the supernatant of transfected MSCs contained miR-21, which could improve the functional recovery of SCI rats and suppress cell death both in vivo and in vitro via the miR-21/PTEN/PDCD4 signaling pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chiodo AE, Scelza WM, Kirshblum SC, Wuermser LA, Ho CH, Priebe MM. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil. 2007;88:S76–83.
Johnson RL, Brooks CA, Whiteneck GG. Cost of traumatic spinal cord injury in a population-based registry. Spinal Cord. 1996;34:470–80.
Di Giovanni S, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI. Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Ann Neurol. 2003;53:454–68.
Davies SJ, Field PM, Raisman G. Regeneration of cut adult axons fails even in the presence of continuous aligned glial pathways. Exp Neurol. 1996;142:203–16.
Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–30.
Neirinckx V, Agirman G, Coste C, Marquet A, Dion V, Rogister B, et al. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther. 2015;6:211.
Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye FB, et al. Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J Neurotrauma. 2017;34:3388–96.
Akcakaya P, Ekelund S, Kolosenko I, Caramuta S, Ozata DM, Xie H, et al. miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int J Oncol. 2011;39:311–8.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Lei P, Li Y, Chen X, Yang S, Zhang J. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 2009;1284:191–201.
Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–9.
Polajeva J, Swartling FJ, Jiang Y, Singh U, Pietras K, Uhrbom L, et al. miRNA-21 is developmentally regulated in mouse brain and is co-expressed with SOX2 in glioma. BMC Cancer. 2012;12:378.
Jiang Y, Zhao S, Ding Y, Nong L, Li H, Gao G, et al. MicroRNA21 promotes neurite outgrowth by regulating PDCD4 in a rat model of spinal cord injury. Mol Med Rep. 2017;16:2522–8.
Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, et al. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–83.
Liu T, Chen G, Sun D, Lei M, Li Y, Zhou C, et al. Exosomes containing miR-21 transfer the characteristic of cisplatin resistance by targeting PTEN and PDCD4 in oral squamous cell carcinoma. Acta Biochim Biophys Sin. 2017;49:808–16.
Fu X, He Y, Wang X, Peng D, Chen X, Li X, et al. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. 2017;8:187.
Matsushita T, Lankford KL, Arroyo EJ, Sasaki M, Neyazi M, Radtke C, et al. Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp Neurol. 2015;267:152–64.
Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226–35.
Sasaki M, Radtke C, Tan AM, Zhao P, Hamada H, Houkin K, et al. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29:14932–41.
Spejo AB, Carvalho JL, Goes AM, Oliveira AL. Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: synapse stability and axonal regeneration. Neuroscience. 2013;250:715–32.
Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–25.
Trams EG, Lauter CJ, Salem N, Jr. Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70.
Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–63.
Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122.
Lai. R, Arslan F, Lee M, Sze N, Choo A, Chen T, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22.
Kordelas L, Rebmann V, Ludwig A, Radtke S, Ruesing J, Doeppner T, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:980–973.
Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–4.
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–64.
Lu XC, Zheng JY, Tang LJ, Huang BS, Li K, Tao Y, et al. MiR-133b Promotes neurite outgrowth by targeting RhoA expression. Cell Physiol Biochem. 2015;35:246–58.
Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D, McGuire TL, et al. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci. 2010;30:1839–55.
Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, et al. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci. 2012;32:17935–47.
Wei C, Li L, Kim IK, Sun P, Gupta S. NF-kappaB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free Radic Res. 2014;48:282–91.
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta. 2010;411:846–52.
Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36.
Lankat-Buttgereit B, Goke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol Cell. 2009;101:309–17.
Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20:669–76.
Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–33.
Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, et al. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. 2010;87:431–9.
Huang JH, Cao Y, Zeng L, Wang G, Cao M, Lu HB, et al. Tetramethylpyrazine enhances functional recovery after contusion spinal cord injury by modulation of MicroRNA-21, FasL, PDCD4 and PTEN expression. Brain Res. 2016;1648:35–45.
Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma. 2013;30:1349–60.
Leslie NR, Foti M. Non-genomic loss of PTEN function in cancer: not in my genes. Trends Pharmacol Sci. 2011;32:131–40.
Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96.
Stahl JM, Cheung M, Sharma A, Trivedi NR, Shanmugam S, Robertson GP. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res. 2003;63:2881–90.
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93:105–17.
Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero D, et al. Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metabol. 2009;29:1903–13.
Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci. 2013;33:15350–61.
Ohtake Y, Park D, Abdul-Muneer PM, Li H, Xu B, Sharma K, et al. The effect of systemic PTEN antagonist peptides on axon growth and functional recovery after spinal cord injury. Biomaterials. 2014;35:4610–26.
Danilov CA, Steward O. Conditional genetic deletion of PTEN after a spinal cord injury enhances regenerative growth of CST axons and motor function recovery in mice. Exp Neurol. 2015;266:147–60.
Acknowledgements
This study was funded by the Shanghai Committee of Science and Technology, China (Grant No. 15411970400) and by the Academic Leaders Training Program of Jingan Health Care Bureau of Shanghai (2016). The Program for Outstanding Medical Academic Leader of Shanghai (Grant No.046), and the Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (Grant no. PWZxq2017‑11)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kang, J., Li, Z., Zhi, Z. et al. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther 26, 491–503 (2019). https://doi.org/10.1038/s41434-019-0101-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-019-0101-8
This article is cited by
-
Exosome-mediated repair of spinal cord injury: a promising therapeutic strategy
Stem Cell Research & Therapy (2024)
-
Spinal Cord Injury: From MicroRNAs to Exosomal MicroRNAs
Molecular Neurobiology (2024)
-
The Importance of Using Exosome-Loaded miRNA for the Treatment of Spinal Cord Injury
Molecular Neurobiology (2023)
-
The Potential of miR-21 in Stem Cell Differentiation and its Application in Tissue Engineering and Regenerative Medicine
Stem Cell Reviews and Reports (2023)
-
Crosstalk between exosomes and autophagy in spinal cord injury: fresh positive target for therapeutic application
Cell and Tissue Research (2023)