Abstract
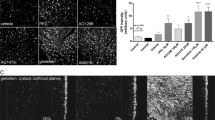
Efficient transduction of the retinal ganglion cells (RGCs) is a prerequisite to maximize therapeutic outcomes in any form of gene therapy for optic neuropathies. Whereas subretinal injection of adeno-associated virus 2 (AAV2) has been well-characterized, the serotype, viral load, and promoter combinations that govern RGC transduction efficiency following intravitreal injection remains poorly understood. We evaluated the transduction efficiency of seven AAV2 serotypes (AAV2/1, AAV2/2, AAV2/4, AAV2/5, AAV2/6, AAV2/8, and AAV2/9) for the RGCs at 4 weeks following intravitreal injection in C57BL/6J mice. Intravitreal injection of 1 × 109 vg of AAV2/2 with eGFP driven by the CMV promoter attained a higher transduction efficiency for the RGCs (60.0 ± 4.2%) compared with the six other AAV2 serotypes with eGFP driven by the same promoter injected at the same viral load ( < 3.0%). Reporter driven by the CAG promoter had a lower transduction efficiency (up to 42.0 ± 5.8%) compared with that driven by the CMV reporter (60.0 ± 4.2%, p ≤ 0.024). There was a viral dose-dependent transduction effect of AAV2/2-CMV-eGFP and the transduction efficiency was 40.2 ± 3.9%, 16.6 ± 4.2%, and 2.6 ± 0.2% when the viral load decreased to 5 × 108 vg, 1 × 108 vg, and 1 × 107 vg, respectively. Optimizing viral serotype, viral load, and promoter construct of AAV2 is important to maximize transgene expression in RGC-targeted gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9.
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr., Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. New Engl J Med. 2008;358:2240–8.
Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA. 2013;110:E517–25.
Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372:1887–97.
Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–60.
MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37.
Edwards TL, Jolly JK, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, et al. Visual acuity after retinal gene therapy for choroideremia. N Engl J Med. 2016;374:1996–8.
McDougald DS, Dine KE, Zezulin AU, Bennett J, Shindler KS. SIRT1 and NRF2 gene transfer mediate distinct neuroprotective effects upon retinal ganglion cell survival and function in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2018;59:1212–20.
Smith CA, Chauhan BC. In vivo imaging of adeno-associated viral vector labelled retinal ganglion cells. Sci Rep. 2018;8:1490.
Hanlon KS, Chadderton N, Palfi A, Blanco Fernandez A, Humphries P, Kenna PF, et al. A novel retinal ganglion cell promoter for utility in AAV vectors. Front Neurosci. 2017;11:521.
Chaffiol A, Caplette R, Jaillard C, Brazhnikova E, Desrosiers M, Dubus E, et al. A new promoter allows optogenetic vision restoration with enhanced sensitivity in macaque retina. Mol Ther: J Am Soc Gene Ther. 2017;25:2546–60.
Cen LP, Liang JJ, Chen JH, Harvey AR, Ng TK, Zhang M, et al. AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience. 2017;343:472–82.
Tshilenge KT, Ameline B, Weber M, Mendes-Madeira A, Nedellec S, Biget M, et al. Vitrectomy before intravitreal injection of AAV2/2 vector promotes efficient transduction of retinal ganglion cells in dogs and nonhuman primates. Hum Gene Ther Methods. 2016;27:122–34.
Mehta ST, Luo X, Park KK, Bixby JL, Lemmon VP. Hyperactivated Stat3 boosts axon regeneration in the CNS. Exp Neurol. 2016;280:115–20.
Yungher BJ, Luo X, Salgueiro Y, Blackmore MG, Park KK. Viral vector-based improvement of optic nerve regeneration: characterization of individual axons’ growth patterns and synaptogenesis in a visual target. Gene Ther. 2015;22:811–21.
Guy J, Qi X, Koilkonda RD, Arguello T, Chou TH, Ruggeri M, et al. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest Ophthalmol Vis Sci. 2009;50:4205–14.
Tomita H, Sugano E, Yawo H, Ishizuka T, Isago H, Narikawa S, et al. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer. Invest Ophthalmol Vis Sci. 2007;48:3821–6.
Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13:1328–41.
MacLaren RE, Buch PK, Smith AJ, Balaggan KS, MacNeil A, Taylor JS, et al. CNTF gene transfer protects ganglion cells in rat retinae undergoing focal injury and branch vessel occlusion. Exp Eye Res. 2006;83:1118–27.
Kwong JM, Caprioli J, Piri N. RNA binding protein with multiple splicing: a new marker for retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:1052–8.
Rodriguez AR, de Sevilla Muller LP, Brecha NC. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol. 2014;522:1411–43.
Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. J Gene Med. 2008;10:375–82.
Leung CK, Weinreb RN, Li ZW, Liu S, Lindsey JD, Choi N, et al. Long-term in vivo imaging and measurement of dendritic shrinkage of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2011;52:1539–47.
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–6.
Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–23.
Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–5.
Wan X, Pei H, Zhao MJ, Yang S, Hu WK, He H, et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy. Sci Rep. 2016;6:21587.
Guy J, Feuer WJ, Davis JL, Porciatti V, Gonzalez PJ, Koilkonda RD, et al. Gene therapy for Leber hereditary optic neuropathy: Low- and medium-dose visual results. Ophthalmology. 2017;124:1621–34.
Feuer WJ, Schiffman JC, Davis JL, Porciatti V, Gonzalez P, Koilkonda RD, et al. Gene therapy for Leber hereditary optic neuropathy: Initial results. Ophthalmology. 2016;123:558–70.
Gray SJ, Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J, et al. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Gene Ther. 2011;22:1143–53.
Acknowledgements
This study was supported by funding from the Hong Kong Research Grant Council General Research Fund 2015/2016 (14101215).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, X., Yung, J., Mak, H. et al. Factors governing the transduction efficiency of adeno-associated virus in the retinal ganglion cells following intravitreal injection. Gene Ther 26, 109–120 (2019). https://doi.org/10.1038/s41434-019-0060-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-019-0060-0
This article is cited by
-
Reduction of retinal ganglion cell death in mouse models of familial dysautonomia using AAV-mediated gene therapy and splicing modulators
Scientific Reports (2023)
-
A 2020 vision of ocular gene therapy
Gene Therapy (2021)