Abstract
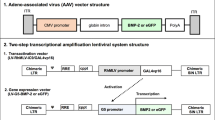
In this study, we developed a lentiviral two-step transcriptional amplification (TSTA) system expressing bone morphogenetic protein-2 (BMP-2) under the control of a GAL4FF transactivator to enhance gene expression and limit toxicity for bone repair applications. To this end human MSCs, isolated from bone marrow or adipose tissue, were transduced overnight with a LV-TSTA system (GAL4FF or GAL4vp16) expressing BMP-2 or GFP and evaluated in vitro for transduction efficiency, mean fluorescence intensity, cell viability, and BMP-2 production. FACS analysis of GFP-transduced MSCs confirmed successful transduction with the GAL4FF+GFP vector. Moreover, ELISA demonstrated abundant BMP-2 production by GAL4FF+BMP2-transduced human MSCs over a period of 8 weeks, with minimal cytotoxicity at all time points. Compared to GAL4vp16, GAL4FF was superior with respect to BMP production at 1, 2, 4, 6, and 8 weeks in BMSCs. In ASCs, GAL4FF was still associated with higher BMP-2 production at weeks 2–8, but this difference was not as prominent as in BMSCs. To our knowledge, this is the first report of GAL4FF-mediated BMP-2 production by human BMSCs and ASCs. Compared to the standard GAL4vp16TSTA vector, GAL4FF was associated with lower cytotoxicity and higher in vitro gene expression in both BMSCs and ASCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Evans CH. Gene delivery to bone. Adv Drug Deliv Rev. 2012;64:1331–40.
Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015;11:234–42.
Pensak MJ, Lieberman JR. Gene therapy for bone regeneration. Curr Pharm Des. 2013;19:3466–73.
Sugiyama O, An DS, Kung SP, Feeley BT, Gamradt S, Liu NQ, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005;11:390–8.
Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–21.
Virk MS, Conduah A, Park SH, Liu N, Sugiyama O, Cuomo A, et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone. 2008;42:921–31.
Seeherman HJ, Li XJ, Bouxsein ML, Wozney JM. RhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J Bone Jt Surg Am. 2010;92:411–26.
Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–10.
Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vector containing factor IX gene: tolerization of factor IX and vector antigens allows for longterm expression. Proc Natl Acad Sci USA. 1995;92:1401–5.
Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–7.
Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA. 2001;98:14595–14600.
Virk MS, Sugiyama O, Park SH, Gambhir SS, Adams DJ, Drissi H, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 2011;19:960–8.
Alaee F, Bartholomae C, Sugiyama O, Virk MS, Drissi H, Wu Q, et al. Biodistribution of LV-TSTA transduced rat bone marrow cells used for “ex-vivo” regional gene therapy for bone repair. Curr Gene Ther. 2015;15:481–91.
Bougioukli S, Sugiyama O, Pannell W, Ortega BA, Tan MH, Tang AH, et al. Gene therapy for bone repair using human cells: superior osteogenic potential of BMP-2 transduced mesenchymal stem cells derived from adipose tissue compared to bone marrow. Hum Gene Ther. 2018;29:507–19.
Sadowski I, Ma J, Triezenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–4.
Gill G, Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988;334:721–4.
Köster RW, Fraser SE. Tracing transgene expression in living zebrafish embryos. Dev Biol. 2001;233:329–46.
Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, et al. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci USA. 2008;105:1255–60.
Franceschi RT, Yang S, Rutherford RB, Krebsbach PH, Zhao M, Wang D. Gene therapy approaches for bone regeneration. Cells Tissues Organs. 2004;176:95–108.
Phillips JE, Gersbach CA, García AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–29.
Carofino BC, Lieberman JR. Gene therapy applications for fracture-healing. J Bone Jt Surg Am. 2008;90:99–110.
Oakes DA, Lieberman JR. Osteoinductive applications of regional gene therapy: ex vivo gene transfer. Clin Orthop Relat Res. 2000;379:S101–112.
Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–8.
Bougioukli S, Jain A, Sugiyama O, Tinsley BA, Tang AH, Tan MH, et al. Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone. 2016;84:93–103.
Pensak M, Hong S, Dukas A, Tinsley B, Drissi H, Tang A, et al. The role of transduced bone marrow cells overexpressing BMP-2 in healing critical-sized defects in a mouse femur. Gene Ther. 2015;22:467–75.
Miyazaki M, Sugiyama O, Tow B, Zou J, Morishita Y, Wei F, et al. The effects of lentiviral gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Spinal Disord Tech. 2008;21:372–9.
Argenton F, Arava Y, Aronheim A, Walker MD. An activation domain of the helix-loop-helix transcription factor E2A shows cell type preference in vivo in microinjected zebrafish embryos. Mol Cell Biol. 1996;16:1714–21.
Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, et al. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Methods. 2007;4:323–6.
Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–9.
Stender S, Murphy M, O’Brien T, Stengaard C, Ulrich-Vinther M, Soballe K, et al. Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur Cell Mater. 2007;13:93–9.
Alaee F, Sugiyama O, Virk MS, Tang Y, Wang B, Lieberman JR. In vitro evaluation of a double-stranded self-complementary adeno-associated virus type2 vector in bone marrow stromal cells for bone healing. Genet Vaccin Ther. 2011;9:4.
Muto A, Kawakami K. Imaging functional neural circuits in zebrafish with a new GCaMP and the Gal4FF-UAS system. Commun Integr Biol. 2011;4:566–8.
Lee O, Tyler CR, Kudoh T. Development of a transient expression assay for detecting environmental oestrogens in zebrafish and medaka embryos. BMC Biotechnol. 2012;12:32.
Moreman J, Lee O, Trznadel M, David A, Kudoh T, Tyler CR. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ Sci Technol. 2017;51:12796–805.
Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2013;79:e50585.
De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–9.
Acknowledgements
This work was supported by a National Institutes of Health grant to J.R.L. [R01AR057076]. The GAL4FF plasmid was kindly provided by Dr. Koichi Kawakami, National Institute of Genetics, Japan. The authors would also like to thank Frank Gonsalves of the Keck Hospital of USC and Judy Yoho of Dr. Yoho’s Cosmetic surgery practice for their valuable assistance in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.B., O.S., R.K.A., R.Y., and D.A.O. have no conflicts to report. J.R.L. has received royalties and has served as a paid consultant for Depuy, is a shareholder in Hip Innovation Technologies, Inc. and has received royalties, financial or material support from Elsevier.
Rights and permissions
About this article
Cite this article
Bougioukli, S., Sugiyama, O., Alluri, R.K. et al. In vitro evaluation of a lentiviral two-step transcriptional amplification system using GAL4FF transactivator for gene therapy applications in bone repair. Gene Ther 25, 260–268 (2018). https://doi.org/10.1038/s41434-018-0024-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-018-0024-9