Abstract
Background/objectives
Previous studies have suggested that lower mean foetal haemoglobin (HbF) levels is associated with an increased risk for developing retinopathy of prematurity (ROP). Lower HbF levels may lead to high oxygen exposure to the developing retina thereby increasing the risk of acute ROP. In this study, we characterize the temporal relationship of HbF levels and the development of ROP.
Subjects/methods
This is a single institution prospective observational cohort study. Preterm infants (born <31 weeks gestational age or <1500 g) with HbF measured at birth (cord blood), 31-, 34-, and 37-weeks post menstrual age (PMA); and at least one ROP exam, were enrolled.
Results
A total of 60 preterm infants (28 females, 47%) were enrolled. At 31-, 34-, 37-weeks PMA, infants with ROP (mild = Type 2 or less severe and severe = Type 1 ROP) had statistically lower percentages of HbF than infants with no ROP (28.2 ± 15 and 9.7 ± 2.9 vs 67.1 ± 29.6; p < 0.0001; 23.3 ± 14.7 and 32.5 vs 60.1 ± 25; p < 0.005; 31.9 ± 15.8 and 41.6 vs 60.2 ± 20.0; p < 0.0019). Infants with HbF levels in the lowest tercile at 31-weeks PMA were 7.6 times more likely to develop mild and severe ROP (95% CI 2.1–24.0, p value = 0.0006) and this risk increased to 12.3 times (95% CI: 2.6-59.0, p value = 0.0017) at 34-weeks PMA.
Conclusions
Low HbF levels at 31- and 34-weeks PMA are associated with significantly increased risk of developing ROP. The decrease in HbF precedes the development of ROP and may be important in its pathogenesis.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is the leading cause of blindness. Early seminal studies of ROP by Michelson, Ashton, and Patz demonstrated that continuous exposure to high oxygen levels was toxic to retinal development in animals and preterm human infants [1,2,3,4]. It has been hypothesized that high supplemental oxygen exposure at birth for these preterm infants leads to vasoconstriction and delay in further retinal vascular development. Subsequent exposure to hypoxia, stimulates the release of angiogenic factors, such as insulin-like growth factor and vascular endothelial growth factor, which are important in the pathogenesis of ROP [5,6,7].
To understand retinal oxygenation and the stimulus for developing ROP, the role of adult haemoglobin (HbA) and foetal haemoglobin (HbF) should be considered. In utero and at term birth, HbF comprises 70–90% of total haemoglobin. HbF differs from HbA in its (1) decreased binding to 2,3 diphosphoglycerate (2, 3 DPG) and therefore higher affinity for oxygen, (2) leftward shift in the oxyhaemoglobin dissociation curve, and (3) steeper oxyhaemoglobin dissociation curve. HbF’s left shift of the oxyhaemoglobin dissociation and steeper oxyhaemoglobin dissociation curve provides a greater oxygen delivery to tissues due to its earlier release of oxygen during hypoxia and small changes in decreased tissue oxygenation.
Prior studies have examined the relationship of HbF levels and risk of ROP development. Erdol et al. reported that blood transfusions for anaemia in preterm infants decreased HbF levels and increased HbA levels [8]. However, the observed changes in HbF and HbA levels were not associated with an increased risk for development of ROP [8]. In contrast, Stutchfield et al. found that preterm infants with lower mean HbF levels from blood transfusions were at increased risk for developing ROP. However, the temporal relationship between HbF levels and interval development of ROP were not explored [9]. Because the angiogenesis phase of retinal development, which starts during the 31st week of gestation has been identified as important for ROP development, the aim of this study was to characterize HbF levels between 31 and 40 weeks of gestation, and to determine whether a decline in HbF levels at specific time points was associated with an increased risk of developing ROP. Identifying HbF as a biomarker for the development of ROP at specific time points during the first few weeks of the preterm infant’s life may provide insight into the pathophysiology of ROP as well as guide clinical examination timing.
Methods
This prospective single institutional observational cohort study was approved by the Johns Hopkins Institutional Review Board. Preterm infants meeting the American Academy of Pediatrics Screening Recommendation for ROP screening were recruited [10]. Written informed consent was obtained from the legal guardian/parent of the infant. Heel blood (0.5 ml) was collected from each preterm infant at 31-, 34-, and 37-weeks post menstrual age (PMA). Previously collected cord blood was analysed when possible. Haemoglobin percentage levels were measured using high-performance liquid chromotography on a D-10 Haemoglobin Testing System (Bio-Rad, Paris, France). ROP examinations were performed by experienced paediatric ophthalmologists (MXR, MC, CK). ROP was defined per the revised International Classification of Retinopathy of Prematurity [11]. The ophthalmic examination was classified as no ROP, mild ROP (Type 2 or less severe ROP), or severe ROP (Type 1 ROP). Standard descriptive statistics were used to describe the study cohort. Analysis of variance was used to explore differences between those who did and did not develop ROP. All statistical analyses were performed on SPSS 22.0 (SPSS, Chicago, IL).
Results
Baseline characteristic of preterm infants with and without ROP
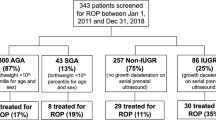
A total of 64 preterm infants were enrolled from 1 September 2017 through 31 December 2018. Four infants were excluded due to not having baseline haemoglobin studies done (three infants were transferred before the 31-week PMA sample could be obtained, one infant had the consent withdrawn prior to 31-weeks PMA). Baseline clinical characteristics are presented in Table 1. There were 45 of 60 (75%) preterm infants with no ROP, 12 of 60 (20%) with mild ROP, and 3 of 60 (5%) with severe ROP. Parity sex, and race were not significantly different among these three groups. Preterm infants who developed any ROP had significantly lower gestational age compared with infants without ROP (p < 0.0001). In addition, preterm infants with ROP had lower birthweight (p = 0.018), more severe medical comorbidities of bronchopulmonary dysplasia, patent ductus arteriosus, and intraventricular haemorrhage, than preterm infants without ROP (p < 0.018).
Infants with ROP have lower fetal haemoglobin levels
Total haemoglobin, which is comprised of HbF and HbA, did not significantly differ among the three groups (no ROP, mild ROP, and severe ROP) at 31-, 34-, and 37-weeks PMA (p > 0.19). However, preterm infants with lower HbF levels had a significantly higher risk of developing ROP (Fig. 1). At 31-weeks PMA, preterm infants who developed mild or severe ROP had significantly lower HbF levels compared with no ROP (28.2%, 9.7%, vs 67.1%, respectively) and higher HbA levels (69.9%, 87.7% vs 31.7%, respectively) compared with those with no ROP (p < 0.0001) (Table 2). This statistically significant difference was observed through 37-weeks PMA (Table 2).
Preterm infants with HbF levels in the lowest tercile at 31-weeks PMA had a hazard ratio 7.6 times greater for the development of ROP (mild and severe) compared with infants in the other terciles (Table 3). Preterm infants with HbA levels in the highest terciles at 31-weeks PMA were 5.5 times more likely to develop ROP (mild and severe) than those in the lower two terciles (Table 3). At 34-weeks PMA, the risk associated with the highest tercile of HbA increased by 1.3-fold. With increasing age beyond 31-weeks PMA, an increased likelihood of developing ROP (mild and severe) was observed with lower HbF and higher HbA levels. At 34-weeks PMA, preterm infants with HbF levels in the lowest tercile were 12.3 times more likely to develop ROP (mild and severe) than those with HbF in the first and second terciles. In addition, at 34-weeks PMA, preterm infants with HbA in the highest tercile were 7.4 times more likely to develop ROP (mild and severe) than those with HbA levels in the lower tercile.
Discussion
While total haemoglobin did not differ among infants with and without ROP, we observed that the (1) ratio of HbF:HbA differed significantly among infants who did and did not develop ROP, and (2) low HbF levels were highly predictive of ROP development. Furthermore, this observed temporal decline in HbF:HbA levels was associated with increased risk for developing ROP. At 31- weeks PMA, preterm infants with lower HbF levels had significantly increased risk of developing ROP and this risk increased by 1.6 times at 34 weeks PMA compared with those with HbF in the first and second tercile. Based on the natural history of ROP progression, the risk of Type 1 ROP is highest between 33 and 36 weeks PMA [12]. Interestingly, we observed a decline in HbF levels in our study cohort interval starting at 31 weeks PMA (2–5 weeks earlier).
Although it is assumed that oxygen delivery is decreased in preterm infants due to the high affinity of HbF, the left shift of the oxyhaemoglobin dissociation curve from high HbF levels to more HbA may still maintain sufficient oxygen delivery to tissues during severe hypoxia. In support of this assumption, Wimberley et al. calculated that with severe hypoxemia (arterial PaO2 < 32 mm Hg and venous PO2 < 10 mm Hg), infants had better tissue oxygen delivery due to a foetal rather than an adult oxyhaemoglobin dissociation curve [13]. Stockman et al. showed that in preterm infants with a right shifted oxyhaemoglobin dissociation curve (HbF < 30%) due to blood transfusion of adult red blood cells, haemoglobin levels fell 2–3 g/dl more than those with left shift (HbF > 60%) before a comparable erythropoietin response occurred [14].
Furthermore, the unique properties of HbF confer clear advantages to oxygen delivery in the preterm infant as compared with HbA. HbF has a higher affinity for oxygen due to its decreased binding to 2, 3 DPG [15]. As a result, HbF has a lower P50 (arterial PaO2 at which haemoglobin is 50% saturated) than HbA, resulting in a leftward shift in the oxyhaemoglobin dissociation curve, and effectively lowering the arterial PaO2 required for the release of oxygen to tissue. Moreover, the HbF oxyhaemoglobin dissociation curve is steeper than that of HbA, resulting in a larger unloading of oxygen to the foetal tissues in response to a small decrease in arterial PaO2.
Subgroup analysis of the Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) highlighted the challenges of oxygen management in preterm infants [16]. SUPPORT showed that despite careful monitoring and regulation of supplemental oxygen, preterm infants experienced increased episodes of intermittent hypoxemia (O2 saturation ≤ 80%) over the first 3 weeks of life [16]. Intermittent hypoxemia has been associated with exacerbation of retinal neovascularisation and neurologic impairment in preterm infants [17,18,19]. Because HbF has better oxygen delivery during hypoxia, we hypothesize that preterm infants with high HbF levels will be protected against these episodes of intermittent hypoxemia, and therefore, be less susceptible to developing ROP.
Based on large multicenter clinical trials such as SUPPORT, the optimal oxygen saturation targeting that balances the morbidity and mortality of oxygen limitation while minimizing ROP development found 90–95% superior to 85–90% regardless of current gestational age [20, 21]. However, as HbF is the primary oxygen transporter in utero and throughout the first 6 months of postnatal life, external factors that decrease HbF levels, such as blood transfusions, may increase the risk of ROP development and other systemic diseases.
Limitations of this study include sampling of HbF at three time points rather than weekly, which would have allowed a more granular measurement of HbF levels. We were unable to obtain weekly samples of HbF because multiple blood draws are a known contributor to anaemia of prematurity. Therefore, study blood draws were limited to medically indicated blood draws (31-, 34-, and 37-weeks) and as determined by the NICU team. Due to the small numbers of infants with severe ROP, we are unable to differentiate the effects of HbF and HbA on the development of mild and severe ROP at 34- and 37-weeks PMA. Despite limiting our time intervals to pre-specified weeks and small number of infants with severe ROP, the findings were sufficiently robust to demonstrate a strong correlation between levels of HbF at 31- and 34-weeks PMA and the development of ROP, as well as an increased risk of ROP, which precedes the development of severe ROP in the natural history studies. An additional limitation is that the more immature infants with higher comorbidities are also those most likely to require transfusions, making individual contributions of these risk factors difficult to assess.
We plan to study factors which influence HbF levels, oxygenation and whether artificially increasing HbF either through neonate to neonate blood transfusions or hydroxyurea can improve local and systemic outcomes of oxygenation in preterm infants and the development of ROP.
Summary
What was known before
-
Prior studies have examined the relationship of HbF levels and risk of ROP development. However, the temporal relationship between HbF levels and interval development of ROP were not explored
What this study adds
-
There is a temporal relationship with the decrease of foetal haemoglobin at 31-weeks PMA and 34-weeks PMA preceeding the onset of ROP.
References
Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br J Ophthalmol. 1953;37:513–20.
Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–432.
IC M. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. Trans Ophthalmol Soc. 1948;68:137–80.
Patz A, Eastham A, Higginbotham DH, Kleh T. Oxygen studies in retrolental fibroplasia. II. The production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953;36:1511–22.
Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Ophthalmol Soc. 1968;66:940–85.
Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–9.
Sonmez K, Drenser KA, Capone A Jr, Trese MT. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology. 2008;115:1065–70. e1061.
Erdol H, Hacioglu D, Kola M, Turk A, Aslan Y. Investigation of the effect of hemoglobin F and A levels on development of retinopathy of prematurity. J AAPOS. 2017;21:136–40.
Stutchfield CJ, Jain A, Odd D, Williams C, Markham R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye. 2017;31:1451–5.
Fierson WM, American Academy of Pediatrics Section on O, American Academy of O, American Association for Pediatric O, Strabismus, American Association of Certified O. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95.
International Committee for the Classification of Retinopathy of P. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Christiansen SP, Dobson V, Quinn GE, Good WV, Tung B, Hardy RJ, et al. Progression of type 2 to type 1 retinopathy of prematurity in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010;128:461–5.
Wimberley PD. Fetal hemoglobin, 2,3-diphosphoglycerate and oxygen transport in the newborn premature infant. Scand J Clin Lab Invest Suppl. 1982;160:1–149.
Stockman JA 3rd, Garcia JF, Oski FA. The anemia of prematurity. Factors governing the erythropoietin response. N Engl J Med. 1977;296:647–50.
Bunn HF, Briehl RW. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970;49:1088–95.
Di Fiore JM, Walsh M, Wrage L, Rich W, Finer N, Carlo WA, et al. Low oxygen saturation target range is associated with increased incidence of intermittent hypoxemia. J Pediatr. 2012;161:1047–52.
Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73.
Poets CF. Intermittent hypoxemia/bradycardia and the developing brain: how much is too much? Commentary on M.B. Schmid et al.: Cerebral oxygenation during intermittent hypoxemia and bradycardia in preterm infants (Neonatology 2015;107:137-146). Neonatology. 2015;107:147–9.
Pillekamp F, Hermann C, Keller T, von Gontard A, Kribs A, Roth B. Factors influencing apnea and bradycardia of prematurity - implications for neurodevelopment. Neonatology. 2007;91:155–61.
Bancalari E, Claure N. Oxygenation targets and outcomes in premature infants. JAMA. 2013;309:2161–2.
Polin RA, Bateman D. Oxygen-saturation targets in preterm infants. N Engl J Med. 2013;368:2141–2.
Funding
This work was supported by the Knights Templar Eye Foundation Career Starter Award to KJ; The Wilmer Eye Institute receives funding from RPB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KJ received a Knights Templar Eye Foundation Career Starter Award. The Knights Templar Eye Foundation did not have a role in the study design, collection and analysis of the data, decision to publish, or preparation of the manuscript. None of the authors have competing financial interests in relation to the work described.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiramongkolchai, K., Repka, M.X., Tian, J. et al. Lower foetal haemoglobin levels at 31- and 34-weeks post menstrual age is associated with the development of retinopathy of prematurity. Eye 35, 659–664 (2021). https://doi.org/10.1038/s41433-020-0938-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0938-5