Abstract
Background/Objectives
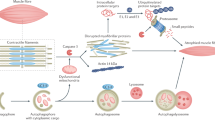
Long-lived proteins and organelles, such as mitochondria and the sarcoplasmic reticulum, are degraded by autophagy. However, the specific role of autophagy in chronic kidney disease (CKD) muscle atrophy is still undefined.
Subjects/Methods
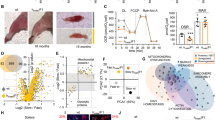
This was a cross-sectional study with 20 subjects and 11 controls. Autophagy induction was studied in human skeletal muscle biopsies from CKD patients and controls by comparing the cross-sectional areas of muscle fibers, protein, and mRNA expression of autophagy-related genes and the appearance of autophagosomes.
Results
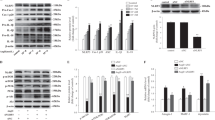
The cross-sectional area of muscle fibers was decreased in CKD patients as compared with the control group. CKD was associated with activated autophagy and mitophagy, as measured by the elevated mRNA and protein expression of BNIP3, (microtubule-associated proteins 1 A/1B light chain 3, also MAP1LC3) LC3, p62, PINK1, and PARKIN in the skeletal muscle and isolated mitochondria of the CKD group. Electron microscopy and immunohistofluorescence analysis showed mitochondrial engulfment by autophagosomes. Mitophagy was further demonstrated by the colocalization of LC3 and p62 puncta with the mitochondrial outer membrane protein TOM20. In addition, degradative FOXO3 (Forkhead box O3) was activated and synthetic mTOR (mammalian target of rapamycin) was inhibited, whereas the upstream mediators VPS34 (class III PI3-kinase) and AKT (protein kinase B, PKB) were activated in CKD patients.
Conclusions
Hyperactive autophagy and mitophagy may play important roles in CKD muscle atrophy. Autophagy was activated by FOXO3 translational factors in the skeletal muscle tissues of CKD patients, which maybe a new way of intervention for CKD muscle atrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008;27:57–64.
Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23:77–90.
Mitch WE. Malnutrition: a frequent misdiagnosis for hemodialysis patients. J Clin Invest. 2002;110:437–9.
Stenvinkel P, Heimburger O, Lindholm B. Wasting, but not malnutrition, predicts cardiovascular mortality in end-stage renal disease. Nephrol Dial Transplant. 2004;19:2181–3.
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51.
Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, et al. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–55.
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–15.
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41.
Romanello V, Sandri M. Mitochondrial quality control and muscle mass maintenance. Front Physiol. 2015;6:422.
Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Cohen AH, et al. Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol. 2012;112:72–8.
Matsumoto N, Ichimura S, Hamaoka T, Osada T, Hattori M, Miyakawa S. Impaired muscle oxygen metabolism in uremic children: improved after renal transplantation. Am J Kidney Dis. 2006;48:473–80.
Verzola D, Procopio V, Sofia A, Villaggio B, Tarroni A, Bonanni A, et al. Apoptosis and myostatin mRNA are upregulated in the skeletal muscle of patients with chronic kidney disease. Kidney Int. 2011;79:773–82.
Conjard A, Ferrier B, Martin M, Caillette A, Carrier H, Baverel G. Effects of chronic renal failure on enzymes of energy metabolism in individual human muscle fibers. J Am Soc Nephrol. 1995;6:68–74.
Yamamoto D, Maki T, Herningtyas EH, Ikeshita N, Shibahara H, Sugiyama Y, et al. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve. 2010;41:819–27.
Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119:2623–33.
Mitsuhashi S, Hatakeyama H, Karahashi M, Koumura T, Nonaka I, Hayashi YK, et al. Muscle choline kinase beta defect causes mitochondrial dysfunction and increased mitophagy. Hum Mol Genet. 2011;20:3841–51.
Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17.
Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–81.
Pyo JO, Nah J, Jung YK. Molecules and their functions in autophagy. Exp Mol Med. 2012;44:73–80.
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–71.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8.
Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–31.
Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803.
Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267–78.
Lazaro RP, Kirshner HS. Proximal muscle weakness in uremia. Case reports and review of the literature. Arch Neurol. 1980;37:555–8.
Clyne N, Jogestrand T, Lins LE, Pehrsson SK, Ekelund LG. Factors limiting physical working capacity in predialytic uraemic patients. Acta Med Scand. 1987;222:183–90.
Haggmark T, Thorstensson A. Fibre types in human abdominal muscles. Acta Physiol Scand. 1979;107:319–25.
Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257:E567–72.
Kouidi E, Albani M, Natsis K, Megalopoulos A, Gigis P, Guiba-Tziampiri O, et al. The effects of exercise training on muscle atrophy in haemodialysis patients. Nephrol Dial Transplant. 1998;13:685–99.
Clyne N, Esbjornsson M, Jansson E, Jogestrand T, Lins LE, Pehrsson SK. Effects of renal failure on skeletal muscle. Nephron. 1993;63:395–9.
Sharma D, Hawkins M, Abramowitz MK. Association of sarcopenia with eGFR and misclassification of obesity in adults with CKD in the United States. Clin J Am Soc Nephrol. 2014;9:2079–88.
Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10:504–16.
Franch HA, Mitch WE. Catabolism in uremia: the impact of metabolic acidosis. J Am Soc Nephrol. 1998;9:S78–81.
Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest. 1996;97:1447–53.
Bosutti A, Toigo G, Ciocchi B, Situlin R, Guarnieri G, Biolo G. Regulation of muscle cathepsin B proteolytic activity in protein-depleted patients with chronic diseases. Clin Nutr. 2002;21:373–8. 34
Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab. 2000;278:E219–25.
Balakrishnan VS, Rao M, Menon V, Gordon PL, Pilichowska M, Castaneda F, et al. Resistance training increases muscle mitochondrial biogenesis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:996–1002.
Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46.
Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem. 2011;149:241–51.
Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5.
Park SJ, Rao M, Menon V, Gordon PL, Pilichowska M, Castaneda F, et al. Mitochondrial fragmentation caused by phenanthroline promotes mitophagy. FEBS Lett. 2012;586:4303–10.
Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–59.
Vila M, Ramonet D, Perier C. Mitochondrial alterations in Parkinson’s disease: new clues. J Neurochem. 2008;107:317–28.
Sugie K, Noguchi S, Kozuka Y, Arikawa-Hirasawa E, Tanaka M, Yan C, et al. Autophagic vacuoles with sarcolemmal features delineate Danon disease and related myopathies. J Neuropathol Exp Neurol. 2005;64:513–22.
Raben N, Hill V, Shea L, Takikita S, Baum R, Mizushima N, et al. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum Mol Genet. 2008;17:3897–908.
Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45:2121–9.
Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med. 2010;16:1313–20.
Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103.
De Palma C, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, et al. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis. 2012;3:e418.
Carmignac V, Svensson M, Körner Z, Elowsson L, Matsumura C, Gawlik KI, et al. Autophagy is increased in laminin alpha2 chain-deficient muscle and its inhibition improves muscle morphology in a mouse model of MDC1A. Hum Mol Genet. 2011;20:4891–902.
Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011;25:99–110.
Lokireddy S, Wijesoma IW, Teng S, Bonala S, Gluckman PD, McFarlane C, et al. The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab. 2012;16:613–24.
Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–83.
Mofarrahi M, Sigala I, Guo Y, Godin R, Davis EC, Petrof BS, et al. Autophagy and skeletal muscles in sepsis. PLoS ONE. 2012;7:e47265.
Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol. 2006;17:1388–94.
Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–6.
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412.
Lacson E Jr, Wang W, Zebrowski B, Wingard R, Hakim RM. Outcomes associated with intradialytic oral nutritional supplements in patients undergoing maintenance hemodialysis: a quality improvement report. Am J Kidney Dis. 2012;60:591–600.
Chertow GM, Ling J, Lew NL, Lazarus JM, Lowrie EG. The association of intradialytic parenteral nutrition administration with survival in hemodialysis patients. Am J Kidney Dis. 1994;24:912–20.
Acknowledgements
We are indebted to the patients, whose participation made this study possible. We thank Zhang Boyan at the Department of Central Laboratory, Eye and ENT Hospital of Fudan University, for technical support for confocal imaging and Chen Xinyu at the Shanghai Jiaotong University School of Medicine for technical support for electron microscopy. We thank the collaborators, who assisted in obtaining the tissue studied in the research, as follows: Xu Mindong at the Department of Nephrology, Tongji University Affiliated Shanghai Yangpu District Central Hospital; Han Guofeng and Hu Weifeng at the Department of Nephrology, No. 455 Hospital of People’s Liberation Army, China; Xi Xiaowei and Chen Yongping at the Department of Obstetrics and Gynecology, Shanghai Jiaotong University Affiliated Shanghai First People’s Hospital; Peng Zhihai at the Department of General Surgery, Shanghai Jiaotong University Affiliated Shanghai First People’s Hospital. We thank Pubsci Limited for editorial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, Y.y., Gu, L.j., Huang, J. et al. CKD autophagy activation and skeletal muscle atrophy—a preliminary study of mitophagy and inflammation. Eur J Clin Nutr 73, 950–960 (2019). https://doi.org/10.1038/s41430-018-0381-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-018-0381-x
This article is cited by
-
Curcumin regulates autophagy through SIRT3-SOD2-ROS signaling pathway to improve quadriceps femoris muscle atrophy in KOA rat model
Scientific Reports (2024)
-
PI3K/AKT/FOXO3a Pathway Induces Muscle Atrophy by Ubiquitin-Proteasome System and Autophagy System in COPD Rat Model
Cell Biochemistry and Biophysics (2024)
-
Mitochondrial dysfunction: roles in skeletal muscle atrophy
Journal of Translational Medicine (2023)
-
Pathophysiological mechanisms leading to muscle loss in chronic kidney disease
Nature Reviews Nephrology (2022)
-
Skeletal Muscle Complications in Chronic Kidney Disease
Current Osteoporosis Reports (2022)