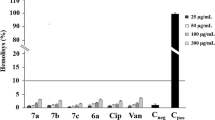
Abstract
The swift spread of infections caused by drug-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), has quickly become a worldwide concern as infections spread from healthcare settings to the wider community. While ferrocenyl chalcones, which are chalcone derivatives with antimicrobial activity, have gained attention from researchers, further study is needed to assess their cytotoxicity. Ten newly developed chalcones, in which ring A was replaced with a ferrocenyl moiety and ring B contained increasing alkyl chain lengths from 1 to 10 carbons, were assessed. Using twofold broth microdilution, the minimum inhibitory concentration (MIC) of five of the ten compounds were lower against Gram-positive organisms (MICs from 0.008 mg ml−1 to 0.063 mg ml−1) than Gram-negative organisms (MICs = 0.125 mg ml−1). These novel ferrocenyl chalcone compounds were effective against three types of clinically isolated drug-resistant S. aureus, including an MRSA, and against other non-resistant clinically isolated and laboratory-adapted Gram-positive bacteria. The same compounds inhibited growth in non-resistant bacteria by potentially obstructing cellular respiration in Gram-positive bacteria. Images obtained through scanning electron microscopy revealed fully lysed bacterial cells once exposed to a selected compound that showed activity. The results indicate that these newly developed compounds could be important antimicrobial agents in the treatment of infections from clinically resistant bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–33.
World Health Organisation. Turning plans into action for antimicrobial resistance (AMR) working paper 2.0: implementation and coordination. Geneva: World Health Organisation; 2019.
Gullberg E, Cao S, Berg OG, Ilbäck C, Sandegren L, Hughes D et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011;7:1–9.
Edwards B, Andini R, Esposito S, Grossi P, Lew D, Mazzei T et al. Treatment options for methicillin-resistant Staphylococcus aureus (MRSA) infection: where are we now? J Glob Antimicrob Resist. 2014;2:133–40.
Normark S, Edlund T, Grundstrom T, Bergstrom S, Wolfwatz H. Escherichia coli K12 mutants hyper producing chromosomal beta lactamase by gene repetitions. J Bacteriol. 1977;132:912–22.
Song S, Berg OG, Roth JR, Andersson DI. Contribution of gene amplification to evolution of increased antibiotic resistance in Salmonella typhimurium. Genetics. 2009;182:1183–95.
Hadley S. Resistant Gram-positive infections: where have we been, where are we now, and where are we going? Clin Ther. 2014;36:1298–302.
WHO. Worldwide country situation analysis: response to antimicrobial resistance. Geneva: WHO; 2015.
Tseng S-H, Lee C-M, Lin T-Y, Chang S-C, Chang F-Y. Emergence and spread of multi-drug resistant organisms: think globally and act locally. J Microbiol Immunol Infect. 2011;44:157–65.
Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L et al. Burden of endemic health-careassociated infection in developing countries: systematic review and meta-analysis. Lancet Infect Dis. 2011;377:228–41.
Newton BA. The properties and mode of action of the polymixins. Bact Rev. 1956;20:14–27.
Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–41.
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–8.
European Centre for Disease Prevention and Control (ECDC). Plasmid-mediated colistin resistance in Enterobacteriaceae. Stockholm: European Centre for Disease Prevention and Control; 2016.
Oelschlaeger P, Ai N, Duprez KT, Welsh WJ, Toney JH. Evolving carbapenemases: can medicinal chemists advance one step ahead of the coming storm? J Med Chem. 2010;53:3013–27.
Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52 SUPPL. 5:397–428.
Kinch MS, Patridge E, Plummer M, Hoyer D. An analysis of FDA-approved drugs for infectious disease: antibacterial agents. Drug Discov Today. 2014;19:1283–7.
Shlaes DM, Spellberg B. Overcoming the challenges to developing new antibiotics. Curr Opin Pharm. 2012;12:522–6.
Cushnie TPT, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 2011;38:99–107.
Attar S, O’Brien Z, Alhaddad H, Golden ML, Calderón-Urrea A. Ferrocenyl chalcones versus organic chalcones: a comparative study of their nematocidal activity. Bioorg Med Chem. 2011;19:2055–73.
Pereira Ávila H, Smânia EDFA, Monache FD, Smânia A. Structure-activity relationship of antibacterial chalcones. Bioorg Med Chem. 2008;16:9790–4.
Moreira Osório T, Delle Monache F, Domeneghini Chiaradia L, Mascarello A, Regina Stumpf T, Roberto Zanetti C et al. Antibacterial activity of chalcones, hydrazones and oxadiazoles against methicillin-resistant Staphylococcus aureus. Bioorg Med Chem Lett. 2012;22:225–30.
Yin B-T, Yan C-Y, Peng X-M, Zhang S-L, Rasheed S, Geng R-X et al. Synthesis and biological evaluation of α-triazolyl chalcones as a new type of potential antimicrobial agents and their interaction with calf thymus DNA and human serum albumin. Eur J Med Chem. 2014;71:148–59.
Suwito H, Kristanti AN, Hayati S, Dewi SR, Amalina I, Puspaningsih NNT. Antimicrobial activities and in silico analysis of methoxy amino chalcone derivatives. Procedia Chem. 2016;18:103–11.
Gopi C, Sastry VG, Dhanaraju MD. Synthesis and spectroscopic characterisation of novel bioactive molecule of 3-(2-substituted) -1H-indol-3-yl) -1-(thiophen-2yl) prop-2-en-1-one chalcone derivatives as effective anti-oxidant and anti-microbial agents. Beni-Suef Univ J Basic Appl Sci. 2016;5:236–43.
Ahmed N, Konduru NK, Owais M. Design, synthesis and antimicrobial activities of novel ferrocenyl and organic chalcone based sulfones and bis-sulfones. Arab J Chem. 2019;12:1879–84.
Crouch LLE. The synthesis of organometallic chalcones. Preston, Lancashire, UK: University of Central Lancashire; 2014.
Haddock BA, Jones CW. Bacterial respiration. Bact Rev. 1977;41:47–99.
Moodley S, Koorbanally NA, Moodley T, Ramjugernath D, Pillay M. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay is a rapid, cheap, screening test for the in vitro anti-tuberculous activity of chalcones. J Microbiol Methods. 2014;104:72–8.
Riss TL, Moravec R, Niles AL, Benink H, Worzella TJ, Minor L. Cell viability assays. In: Assay guidance manual. Bethesda, MD, Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2013.
Wang H, Cheng H, Wang F, Wei D, Wang X. An improved 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay for evaluating the viability of Escherichia coli cells. J Microbiol Methods. 2010;82:330–3.
Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother. 2010;54:3132–42.
Zhao L, Zhang H, Hao T, Li S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015;187:370–7.
Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48 suppl. 1:5–16.
Medu EO. Examination of the antibacterial activities of some semi-synthetic chalcone-derivatives alone and in combination with polymyxin B. Aberdeen, Scotland, UK: Robert Gordon University; 2013.
Basch H, Gadebusch HH, Brunswick N. In vitro antimicrobial activity of dimethylsulfoxide. Appl Microbiol. 1968;16:1953–4.
Hassan AS. The antibacterial activity of dimethyl sulfoxide (DMSO) with and without of Some ligand complexes of the transitional metal ions of ethyl coumarin against bacteria isolate from burn and wound infection. J Nat Sci Res. 2014;4:106–11.
Bolla JM, Alibert-Franco S, Handzlik J, Chevalier J, Mahamoud A, Boyer G et al. Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Lett. 2011;585:1682–90.
Fux CA, Shirtliff M, Stoodley P, Costerton JW. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 2005;13:58–63.
Li J, Wang G, Zhu H, Zhang M, Zheng X, Di Z et al. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci Rep. 2014;4:4359.
Zengin H, Baysal AH. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19:17773–98.
Kunthalert D, Baothong S, Khetkam P, Chokchaisiri S, Suksamrarn A. A chalcone with potent inhibiting activity against biofilm formation by nontypeable haemophilus influenzae. Microbiol Immunol. 2014;58:581–9.
Kowalski K, Koceva-Chy A, Szczupak L, Hikisz P, Bernasińska J, Rajnisz A et al. Ferrocenylvinyl-flavones: synthesis, structure, anticancer and antibacterial activity studies. J Organomet Chem. 2013;741–742:153–61.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The ferrocenyl chalcone compounds were provided by L. Crouch and R. Smith (University of Central Lancashire) and the clinical isolates were donated by M. Collins (Chesterfield Royal Hospital NHS Foundation Trust).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Henry, E.J., Bird, S.J., Gowland, P. et al. Ferrocenyl chalcone derivatives as possible antimicrobial agents. J Antibiot 73, 299–308 (2020). https://doi.org/10.1038/s41429-020-0280-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-0280-y