Abstract
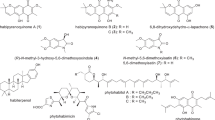
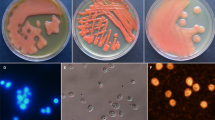
In actinomycetes, many secondary metabolite biosynthetic genes are not expressed under typical laboratory culture conditions and various efforts have been made to activate these dormant genes. In this study, we focused on high-temperature culture. First, we examined the thermotolerance of 3160 actinomycete strains from our laboratory culture collection and selected 57 thermotolerant actinomycetes that grew well at 45 °C. These 57 thermotolerant actinomycetes were cultured for 5 days in liquid medium at both 30 °C and 45 °C. Culture broths were extracted with 1-butanol, and each extract was subjected to LC/MS analysis. The metabolic profiles of each strain were compared between the 30 °C and 45 °C cultures. We found that almost half of these thermotolerant actinomycetes produced secondary metabolites that were detected only in the 45 °C culture. This result suggests that high-temperature culture induces the production of dormant secondary metabolites. These compounds were named “heat shock metabolites (HSMs).” To examine HSM production in more detail, 18 strains were selected at random from the initial 57 strains and cultivated in six different media at 30 °C and 45 °C; as before, metabolic profiles of each strain in each medium were compared between the 30 °C and 45 °C cultures. From this analysis, we found a total of 131 HSMs. We identified several angucycline-related compounds as HSMs from two thermotolerant Streptomyces species. Furthermore, we discovered a new compound, murecholamide, as an HSM from thermotolerant Streptomyces sp. AY2. We propose that high-temperature culture of actinomycetes is a convenient method for activating dormant secondary metabolite biosynthetic genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dictionary of natural products on DVD, version 23.1. London, UK: Chapman & Hall/CRC;2014.
Ohnishi Y, et al. Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J Bacteriol. 2008;190:4050–60.
Bentley SD, et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature. 2002;417:141–7.
Ikeda H, Kazuo SY, Omura S. Genome mining of the Streptomyces avermitilis genome and development of genome-minimized hosts for heterologous expression of biosynthetic gene clusters. J Ind Microbiol Biotechnol. 2014;41:233–50.
van Keulen G, Dyson PJ. Production of specialized metabolites by Streptomyces coelicolor A3(2). Adv Appl Microbiol. 2014;89:217–66.
Katz L, Baltz RH. Natural product discovery: past, present, and future. J Ind Microbiol Biotechnol. 2016;43:155–76.
Ochi K, et al. Ribosome engineering and secondary metabolite production. Adv Appl Microbiol. 2004;56:155–84.
Onaka H. Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J Antibiot. 2017;70:865–70.
Onaka H, Mori Y, Igarashi Y, Furumai T. Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl Environ Microbiol. 2011;77:400–6.
Asamizu S, Ozaki T, Teramoto K, Satoh K, Onaka H. Killing of mycolic acid-containing bacteria aborted induction of antibiotic production by Streptomyces in combined-culture. PLoS ONE. 2015;10:e0142372.
Keskar SS, Srinivasan MC, Deshpande VV. Chemical modification of a xylanase from a thermotolerant Streptomyces. Evidence for essential tryptophan and cysteine residues at the active site. Biochem J. 1989;261:49–55.
Doull JL, Ayer SW, Singh AK, Thibault P. Production of a novel polyketide antibiotic, jadomycin B, by Streptomyces venezuelae following heat shock. J Antibiot. 1993;46:869–71.
Wei ZH, Wu H, Bai L, Deng Z, Zhong JJ. Temperature shift-induced reactive oxygen species enhanced validamycin A production in fermentation of Streptomyces hygroscopicus 5008. Bioprocess Biosyst Eng. 2012;35:1309–16.
Akiyama H, Oku N, Harunari E, Panbangred W, Igarashi Y. Complete NMR assignment and absolute configuration of k4610422, a norditerpenoid inhibitor of testosterone-5α-reductase originally from Streptosporangium: rediscovery from a thermophilic Actinomadura. J Antibiot. 2019. https://doi.org/10.1038/s41429-019-0231-7.
Teta R, et al. Thermoactinoamide A, an antibiotic lipophilic cyclopeptide from the Icelandic thermophilic bacterium Thermoactinomyces vulgaris. J Nat Prod. 2017;80:2530–5.
Hwang BK, Lim SW, Kim BS, Lee JY, Moon SS. Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Appl Environ Chem. 2001;67:3739–45.
Kern DL, Schaumberg JP, Hokanson GC, French JC. PD 116,779, a new antitumor antibiotic of the benz[a]anthraquinone class. J Antibiot. 1986;39:469–70.
Kesenheimer C, Kalogerakis A, Meissner A, Groth U. The cobalt way to angucyclinones: asymmetric total synthesis of the antibiotics (+)-rubiginone B2, (−)-tetrangomycin, and (−)-8-O-methyltetrangomycin. Chemistry. 2010;16:8805–21.
Yamashita N, Harada T, Shin-ya K, Seto H. 6-Hydroxytetrangulol, a new CPP32 protease inducer produced by Streptomyces sp. J Antibiot. 1998;51:79–81.
Kallio P, Liu Z, Mäntsälä P, Niemi J, Metsä-Ketelä M. Sequential action of two flavoenzymes, PgaE and PgaM, in angucycline biosynthesis: chemoenzymatic synthesis of gaudimycin C. Chem Biol. 2008;15:157–66.
Palmu K, Ishida K, Mäntsälä P, Hertweck C, Metsä-Ketelä M. Artificial reconstruction of two cryptic angucycline antibiotic biosynthetic pathways. Chembiochem. 2007;8:1577–84.
Rohr J, et al. Metabolic products of microorganisms. 249. Tetracenomycins B3 and D3, key intermediates of the elloramycin and tetracenomycin C biosynthesis. J Antibiot. 1988;41:1066–73.
Gorajana A, et al. Resistoflavine, cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Microbiol Res. 2007;162:322–7.
Kock I, Maskey RP, Biabani MA, Helmke E, Laatsch H. 1-Hydroxy-1-norresistomycin and resistoflavin methyl ether: new antibiotics from marine-derived streptomycetes. J Antibiot. 2005;58:530–4.
Wang W, et al. Angucyclines as signals modulate the behaviors of Streptomyces coelicolor. Proc Natl Acad Sci USA. 2014;111:5688–93.
Barona-Gómez F, Wong U, Giannakopulos AE, Derrick PJ, Challis GL. Identification of a cluster of genes that directs desferrioxamine biosynthesis in Streptomyces coelicolor M145. J Am Chem Soc. 2004;126:16282–3.
Lee HS, et al. Cyclic peptides of the nocardamine class from a marine-derived bacterium of the genus Streptomyces. J Nat Prod. 2005;68:623–5.
Tokyo Chemical Industry Co., Ltd., Japan. https://www.tcichemicals.com/ja/jp/index.html (Accessed on Sep 29, 2019).
Drzyzga O, Fernández de las Heras L, Morales V, Navarro Llorens JM, Perera J. Cholesterol degradation by Gordonia cholesterolivorans. Appl Environ Microbiol. 2011;77:4802–10.
Nakae K, et al. Migrastatin, a new inhibitor of tumor cell migration from Streptomyces sp. MK929-43F1. Taxonomy, fermentation, isolation and biological activities. J Antibiot. 2000;53:1130–6.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research in Innovative Areas (Grant JP17H06401 to MI) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Saito, S., Kato, W., Ikeda, H. et al. Discovery of “heat shock metabolites” produced by thermotolerant actinomycetes in high-temperature culture. J Antibiot 73, 203–210 (2020). https://doi.org/10.1038/s41429-020-0279-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-0279-4
This article is cited by
-
Winners of the 2021 JA Ōmura Awards for excellence
The Journal of Antibiotics (2023)
-
Heat stress enhanced perylenequinones biosynthesis of Shiraia sp. Slf14(w) through nitric oxide formation
Applied Microbiology and Biotechnology (2023)
-
Noaoxazole, a new heat shock metabolite produced by thermotolerant Streptomyces sp. HR41
The Journal of Antibiotics (2022)