Abstract
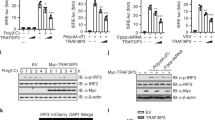
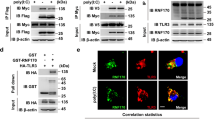
Recognition of viral dsRNA by Toll-like receptor 3 (TLR3) leads to the induction of downstream antiviral effectors and the innate antiviral immune response. Here, we identified the zinc-finger FYVE domain-containing protein ZFYVE1, a guanylate-binding protein (GBP), as a positive regulator of TLR3-mediated signaling. Overexpression of ZFYVE1 promoted the transcription of downstream antiviral genes upon stimulation with the synthetic TLR3 ligand poly(I:C). Conversely, ZFYVE1 deficiency had the opposite effect. Zfyve1−/− mice were less susceptible than wild-type mice to inflammatory death induced by poly(I:C) but not LPS. ZFYVE1 was associated with TLR3, and the FYVE domain of ZFYVE1 and the ectodomain of TLR3 were shown to be responsible for their interaction. ZFYVE1 was bound to poly(I:C) and increased the binding affinity of TLR3 to poly(I:C). These findings suggest that ZFYVE1 plays an important role in the TLR3-mediated innate immune and inflammatory responses by promoting the ligand binding of TLR3.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Yang, Y. et al. The RNA-binding protein Mex3B is a coreceptor of Toll-like receptor 3 in innate antiviral response. Cell Res. 26, 288–303 (2016).
Matsumoto, M. et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171, 3154–3162 (2003).
Liu, L. et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320, 379–381 (2008).
Bell, J. K., Askins, J., Hall, P. R., Davies, D. R. & Segal, D. M. The dsRNA binding site of human Toll-like receptor 3. Proc. Natl. Acad. Sci. USA 103, 8792–8797 (2006).
de Bouteiller, O. et al. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J. Biol. Chem. 280, 38133–38145 (2005).
Wang, Y., Liu, L., Davies, D. R. & Segal, D. M. Dimerization of Toll-like receptor 3 (TLR3) is required for ligand binding. J. Biol. Chem. 285, 36836–36841 (2010).
Yamashita, M. et al. Epidermal growth factor receptor is essential for Toll-like receptor 3 signaling. Sci. Signal. 5, ra50 (2012).
Garcia-Cattaneo, A. et al. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc. Natl. Acad. Sci. USA 109, 9053–9058 (2012).
Qi, R., Singh, D. & Kao, C. C. Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization. J. Biol. Chem. 287, 32617–32629 (2012).
Toscano, F. et al. Cleaved/associated TLR3 represents the primary form of the signaling receptor. J. Immunol. 190, 764–773 (2013).
Zhu, S., Wang, G., Lei, X. & Flavell, R. A. Mex3B: a coreceptor to present dsRNA to TLR3. Cell Res. 26, 391–392 (2016).
Johnsen, I. B. et al. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 25, 3335–3346 (2006).
Hu, M. M. & Shu, H. B. Multifaceted roles of TRIM38 in innate immune and inflammatory responses. Cell Mol. Immunol. 14, 331–338 (2017).
Luo, W. W. & Shu, H. B. Delicate regulation of the cGAS-MITA-mediated innate immune response. Cell Mol. Immunol. 15, 666–675 (2018).
Hu, Y. H. et al. WDFY1 mediates TLR3/4 signaling by recruiting TRIF. EMBO Rep. 16, 447–455 (2015).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Ridley, S. H. et al. FENS-1 and DFCP1 are FYVE domain-containing proteins with distinct functions in the endosomal and Golgi compartments. J. Cell Sci. 114, 3991–4000 (2001).
Nanao, T., Koike, M., Yamaguchi, J., Sasaki, M. & Uchiyama, Y. Cellular localization and tissue distribution of endogenous DFCP1 protein. Biomed. Res. 36, 121–133 (2015).
He, J. et al. Membrane insertion of the FYVE domain is modulated by pH. Proteins 76, 852–860 (2009).
Cheung, P. C., Trinkle-Mulcahy, L., Cohen, P. & Lucocq, J. M. Characterization of a novel phosphatidylinositol 3-phosphate-binding protein containing two FYVE fingers in tandem that is targeted to the Golgi. Biochem. J. 355, 113–121 (2001).
Verma, R. & Bharti, K. Toll like receptor 3 and viral infections of nervous system. J. Neurol. Sci. 372, 40–48 (2017).
Luo, W. W. et al. iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nat. Immunol. 17, 1057–1066 (2016).
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008).
Lian, H. et al. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA sensing and activation of the RIG-I-like receptors. Immunity 49, 438–448 (2018).
Liu, Y. et al. ZDHHC11 modulates innate immune response to DNA virus by mediating MITA-IRF3 association. Cell. Mol. Immunol. 15, 907–916 (2018).
Lin, H. et al. MARCH3 attenuates IL-1beta-triggered inflammation by mediating K48-linked polyubiquitination and degradation of IL-1RI. Proc. Natl. Acad. Sci. USA 115, 12483–12488 (2018).
Jiang, L. Q. et al. IFITM3 inhibits virus-triggered induction of type I interferon by mediating autophagosome-dependent degradation of IRF3. Cell. Mol. Immunol. 15, 858–867 (2018).
Hu, M. M. et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity 45, 555–569 (2016).
Yang, Q. et al. TRIM32-TAX1BP1-dependent selective autophagic degradation of TRIF negatively regulates TLR3/4-mediated innate immune responses. PLoS Pathog. 13, e1006600 (2017).
Lian, H. et al. ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat. Commun. 9, 3349 (2018).
Acknowledgements
This work was supported by grants from the State Key R&D Program of China (2017YFA0505800, 2016YFA0502102 and 2018YFA0800700), the National Natural Science Foundation of China (31830024, 31630045, 31870870, 31800728, 31771555, and 31671418), the China Postdoctoral Science Foundation (2017M620334), the Fundamental Research Funds for the Central Universities (2042019kf0204), and the Natural Science Foundation of Hubei Province (2018CFA016).
Author information
Authors and Affiliations
Contributions
H.-B.S. and X.Z. designed the study; X.Z., L.F., W.-H.X., X.W., and Y.-D.D. performed the experiments; H.-B.S., C.-Q.L., Y.Z., and X.Z. analyzed the data; and H.-B.S., C.-Q.L., and X.Z. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhong, X., Feng, L., Xu, WH. et al. The zinc-finger protein ZFYVE1 modulates TLR3-mediated signaling by facilitating TLR3 ligand binding. Cell Mol Immunol 17, 741–752 (2020). https://doi.org/10.1038/s41423-019-0265-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0265-6
Keywords
This article is cited by
-
Structures and biological functions of zinc finger proteins and their roles in hepatocellular carcinoma
Biomarker Research (2022)