Abstract
Inflammation of the nervous system (neuroinflammation) is now recognized as a hallmark of virtually all neurological disorders. In neuroinflammatory conditions such as multiple sclerosis, there is prominent infiltration and a long-lasting representation of various leukocyte subsets in the central nervous system (CNS) parenchyma. Even in classic neurodegenerative disorders, where such immense inflammatory infiltrates are absent, there is still evidence of activated CNS-intrinsic microglia. The consequences of excessive and uncontrolled neuroinflammation are injury and death to neural elements, which manifest as a heterogeneous set of neurological symptoms. However, it is now readily acknowledged, due to instructive studies from the peripheral nervous system and a large body of CNS literature, that aspects of the neuroinflammatory response can be beneficial for CNS outcomes. The recognized benefits of inflammation to the CNS include the preservation of CNS constituents (neuroprotection), the proliferation and maturation of various neural precursor populations, axonal regeneration, and the reformation of myelin on denuded axons. Herein, we highlight the benefits of neuroinflammation in fostering CNS recovery after neural injury using examples from multiple sclerosis, traumatic spinal cord injury, stroke, and Alzheimer’s disease. We focus on CNS regenerative responses, such as neurogenesis, axonal regeneration, and remyelination, and discuss the mechanisms by which neuroinflammation is pro-regenerative for the CNS. Finally, we highlight treatment strategies that harness the benefits of neuroinflammation for CNS regenerative responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Takeuchi, H. Neurotoxicity by microglia: mechanisms and potential therapeutic strategy. Clin. Exp. Neuroimmunol. 1, 12–21 (2010).
Yong, V. W. Inflammation in neurological disorders: a help or a hindrance? Neuroscientist 16, 408–420 (2010).
Czeh, M., Gressens, P. & Kaindl, A. M. The yin and yang of microglia. Dev. Neurosci. 33, 199–209 (2011).
Kerschensteiner, M., Meinl, E. & Hohlfeld, R. Neuro-immune crosstalk in CNS diseases. Neuroscience 158, 1122–1132 (2009).
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C. & Gage, F. H. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010).
Thompson, A. J., Baranzini, S. E., Geurts, J., Hemmer, B. & Ciccarelli, O. Multiple sclerosis. Lancet 391, 1622–1636 (2018).
Faroni, A., Mobasseri, S. A., Kingham, P. J. & Reid, A. J. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 82-83, 160–167 (2015).
Schwartz, M., Moalem, G., Leibowitz-Amit, R. & Cohen, I. R. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 22, 295–299 (1999).
Bollaerts, I., Van Houcke, J., Andries, L., De Groef, L. & Moons, L. Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediat. Inflamm. 2017, 9478542 (2017).
Labzin, L. I., Heneka, M. T. & Latz, E. Innate immunity and neurodegeneration. Annu. Rev. Med. 69, 437–449 (2018).
Norris, G. T. & Kipnis, J. Immune cells and CNS physiology: microglia and beyond. J. Exp. Med. 216, 60–70 (2019).
Ziv, Y. et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 9, 268–275 (2006).
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Rolls, A. et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 9, 1081–1088 (2007).
Walton, N. M. et al. Microglia instruct subventricular zone neurogenesis. Glia 54, 815–825 (2006).
Yuan, J. et al. M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARgamma signaling pathway. Oncotarget 8, 19855–19865 (2017).
Danilov, A. I. et al. Neurogenesis in the adult spinal cord in an experimental model of multiple sclerosis. Eur. J. Neurosci. 23, 394–400 (2006).
Imitola, J. et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA 101, 18117–18122 (2004).
Lei, C., Wu, B., Cao, T., Liu, M. & Hao, Z. Brain recovery mediated by toll-like receptor 4 in rats after intracerebral hemorrhage. Brain Res. 1632, 1–8 (2016).
Baron, R. et al. IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J. 22, 2843–2852 (2008).
Bosak, V., Murata, K., Bludau, O. & Brand, M. Role of the immune response in initiating central nervous system regeneration in vertebrates: learning from the fish. Int. J. Dev. Biol. 62, 403–417 (2018).
Dietrich, J. et al. Bone marrow drives central nervous system regeneration after radiation injury. J. Clin. Invest. 128, 2651 (2018).
David, S., Bouchard, C., Tsatas, O. & Giftochristos, N. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron 5, 463–469 (1990).
Prewitt, C. M., Niesman, I. R., Kane, C. J. & Houle, J. D. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp. Neurol. 148, 433–443 (1997).
Popovich, P. G. et al. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 158, 351–365 (1999).
Barrette, B. et al. Requirement of myeloid cells for axon regeneration. J. Neurosci. 28, 9363–9376 (2008).
O’Shea, T. M., Burda, J. E. & Sofroniew, M. V. Cell biology of spinal cord injury and repair. J. Clin. Invest. 127, 3259–3270 (2017).
Yin, Y. et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 9, 843–852 (2006).
Yin, Y. et al. Oncomodulin links inflammation to optic nerve regeneration. Proc. Natl. Acad. Sci. USA 106, 19587–19592 (2009).
Chagas, L. D. S. et al. Rapid plasticity of intact axons following a lesion to the visual pathways during early brain development is triggered by microglial activation. Exp. Neurol. 311, 148–161 (2019).
Chen, Q., Smith, G. M. & Shine, H. D. Immune activation is required for NT-3-induced axonal plasticity in chronic spinal cord injury. Exp. Neurol. 209, 497–509 (2008).
Torres-Espin, A. et al. Eliciting inflammation enables successful rehabilitative training in chronic spinal cord injury. Brain 141, 1946–1962 (2018).
Mishra, M. K. & Yong, V. W. Myeloid cells—targets of medication in multiple sclerosis. Nat. Rev. Neurol. 12, 539–551 (2016).
Kigerl, K. A. et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 (2009).
Ma, S. F. et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav. Immun. 45, 157–170 (2015).
Francos-Quijorna, I., Amo-Aparicio, J., Martinez-Muriana, A. & Lopez-Vales, R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia 64, 2079–2092 (2016).
Gadani, S. P., Walsh, J. T., Smirnov, I., Zheng, J. & Kipnis, J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85, 703–709 (2015).
Hauben, E. et al. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J. Neurosci. 20, 6421–6430 (2000).
Ishii, H. et al. Adoptive transfer of Th1-conditioned lymphocytes promotes axonal remodeling and functional recovery after spinal cord injury. Cell Death Dis. 3, e363 (2012).
Schwartz, M. & Raposo, C. Protective autoimmunity: a unifying model for the immune network involved in CNS repair. Neuroscientist 20, 343–358 (2014).
Kipnis, J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771 (2016).
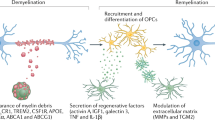
Goldstein, E. Z., Church, J. S., Hesp, Z. C., Popovich, P. G. & McTigue, D. M. A silver lining of neuroinflammation: beneficial effects on myelination. Exp. Neurol. 283, 550–559 (2016).
Rawji, K. S., Mishra, M. K. & Yong, V. W. Regenerative capacity of macrophages for remyelination. Front. Cell Dev. Biol. 4, 47 (2016).
Triarhou, L. C. & Herndon, R. M. Effect of macrophage inactivation on the neuropathology of lysolecithin-induced demyelination. Br. J. Exp. Pathol. 66, 293–301 (1985).
Kotter, M. R., Setzu, A., Sim, F. J., Van Rooijen, N. & Franklin, R. J. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia 35, 204–212 (2001).
Mason, J. L., Suzuki, K., Chaplin, D. D. & Matsushima, G. K. Interleukin-1beta promotes repair of the CNS. J. Neurosci. 21, 7046–7052 (2001).
Arnett, H. A. et al. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 4, 1116–1122 (2001).
Setzu, A. et al. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia 54, 297–303 (2006).
Glezer, I., Lapointe, A. & Rivest, S. Innate immunity triggers oligodendrocyte progenitor reactivity and confines damages to brain injuries. FASEB J. 20, 750–752 (2006).
Butovsky, O. et al. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J. Clin. Invest. 116, 905–915 (2006).
Laflamme, N. et al. mCSF-induced microglial activation prevents myelin loss and promotes its repair in a mouse model of multiple sclerosis. Front. Cell Neurosci. 12, 178 (2018).
Miron, V. E. & Franklin, R. J. Macrophages and CNS remyelination. J. Neurochem. 130, 165–171 (2014).
Miron, V. E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013).
Wolswijk, G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 125, 338–349 (2002).
Patani, R., Balaratnam, M., Vora, A. & Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 33, 277–287 (2007).
Bieber, A. J., Kerr, S. & Rodriguez, M. Efficient central nervous system remyelination requires T cells. Ann. Neurol. 53, 680–684 (2003).
Hvilsted Nielsen, H., Toft-Hansen, H., Lambertsen, K. L., Owens, T. & Finsen, B. Stimulation of adult oligodendrogenesis by myelin-specific T cells. Am. J. Pathol. 179, 2028–2041 (2011).
Baxi, E. G., et al. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J. Neurosci. 35, 8626–8639 (2015).
Zhang, Y. et al. Glatiramer acetate-reactive T lymphocytes regulate oligodendrocyte progenitor cell number in vitro: role of IGF-2. J. Neuroimmunol. 227, 71–79 (2010).
Dombrowski, Y. et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 20, 674–680 (2017).
Yong, V. W. & Rivest, S. Taking advantage of the systemic immune system to cure brain diseases. Neuron 64, 55–60 (2009).
Sousa-Victor, P., Jasper, H. & Neves, J. Trophic factors in inflammation and regeneration: the role of MANF and CDNF. Front. Physiol. 9, 1629 (2018).
O’Donnell, S. L., Frederick, T. J., Krady, J. K., Vannucci, S. J. & Wood, T. L. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia 39, 85–97 (2002).
Higashiyama, S., Abraham, J. A., Miller, J., Fiddes, J. C. & Klagsbrun, M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251, 936–939 (1991).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
McMorris, F. A., Smith, T. M., DeSalvo, S. & Furlanetto, R. W. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc. Natl. Acad. Sci. USA 83, 822–826 (1986).
Scafidi, J. et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature 506, 230–234 (2014).
Woodruff, R. H., Fruttiger, M., Richardson, W. D. & Franklin, R. J. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 25, 252–262 (2004).
Armstrong, R. C., Le, T. Q., Frost, E. E., Borke, R. C. & Vana, A. C. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J. Neurosci. 22, 8574–8585 (2002).
Yuen, T. J. et al. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain 136, 1035–1047 (2013).
Anderson, M. A. et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018).
Giera, S., et al. Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ADGRG1 in oligodendrocyte precursor cells. Elife 7, e33385 (2018).
Kotter, M. R., Zhao, C., van Rooijen, N. & Franklin, R. J. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol. Dis. 18, 166–175 (2005).
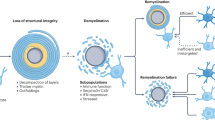
Neumann, H., Kotter, M. R. & Franklin, R. J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132, 288–295 (2009).
Rawji, K. S. et al. Deficient surveillance and phagocytic activity of myeloid cells within demyelinated lesions in aging mice visualized by ex vivo live multiphoton imaging. J. Neurosci. 38, 1973–1988 (2018).
Kotter, M. R., Li, W. W., Zhao, C. & Franklin, R. J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 26, 328–332 (2006).
Larsen, P. H., Wells, J. E., Stallcup, W. B., Opdenakker, G. & Yong, V. W. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J. Neurosci. 23, 11127–11135 (2003).
Siebert, J. R. & Osterhout, D. J. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J. Neurochem. 119, 176–188 (2011).
Lau, L. W. et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 72, 419–432 (2012).
Keough, M. B. et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat. Commun. 7, 11312 (2016).
Morgenstern, D. A., Asher, R. A. & Fawcett, J. W. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain. Res. 137, 313–332 (2002).
Tran, A. P., Warren, P. M. & Silver, J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 98, 881–917 (2018).
Stephenson, E. L. & Yong, V. W. Proinflammatory roles of chondroitin sulfate proteoglycans in disorders of the central nervous system. Matrix Biol. 71-72, 432–442 (2018).
Simard, A. R., Soulet, D., Gowing, G., Julien, J. P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49, 489–502 (2006).
Naert, G. & Rivest, S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 31, 6208–6220 (2011).
Michaud, J. P. et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc. Natl. Acad. Sci. USA 110, 1941–1946 (2013).
ElAli, A. & Rivest, S. Microglia in Alzheimer’s disease: a multifaceted relationship. Brain Behav. Immun. 55, 138–150 (2016).
Sarlus, H. & Heneka, M. T. Microglia in Alzheimer’s disease. J. Clin. Invest. 127, 3240–3249 (2017).
Hamelin, L. et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain 141, 1855–1870 (2018).
Stephenson, E., Nathoo, N., Mahjoub, Y., Dunn, J. F. & Yong, V. W. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat. Rev. Neurol. 10, 459–468 (2014).
Corna, G. et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 95, 1814–1822 (2010).
Recalcati, S. et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur. J. Immunol. 40, 824–835 (2010).
Schonberg, D. L. et al. Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to NG2 cells in vivo. J. Neurosci. 32, 5374–5384 (2012).
Zarruk, J. G. et al. Expression of iron homeostasis proteins in the spinal cord in experimental autoimmune encephalomyelitis and their implications for iron accumulation. Neurobiol. Dis. 81, 93–107 (2015).
Kroner, A. et al. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83, 1098–1116 (2014).
Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
Rinholm, J. E. et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 31, 538–548 (2011).
Shechter, R. & Schwartz, M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J. Pathol. 229, 332–346 (2013).
Yong, V. W. Differential mechanisms of action of interferon-beta and glatiramer aetate in MS. Neurology 59, 802–808 (2002).
Lalive, P. H. et al. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs 25, 401–414 (2011).
Aharoni, R. Immunomodulation neuroprotection and remyelination—the fundamental therapeutic effects of glatiramer acetate: a critical review. J. Autoimmun. 54, 81–92 (2014).
Aharoni, R. et al. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc. Natl. Acad. Sci. USA 102, 19045–19050 (2005).
From, R. et al. Oligodendrogenesis and myelinogenesis during postnatal development effect of glatiramer acetate. Glia 62, 649–665 (2014).
Skihar, V. et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc. Natl. Acad. Sci. USA 106, 17992–17997 (2009).
Cohen, M. et al. Chronic exposure to TGFbeta1 regulates myeloid cell inflammatory response in an IRF7-dependent manner. EMBO J. 33, 2906–2921 (2014).
Yamanaka, M. et al. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 32, 17321–17331 (2012).
Zhang, B. et al. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J. Neuroinflamm. 12, 218 (2015).
Gensel, J. C., Kopper, T. J., Zhang, B., Orr, M. B. & Bailey, W. M. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci. Rep. 7, 40144 (2017).
Zabala, A., et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 10, e8743 (2018).
Doring, A. et al. Stimulation of monocytes, macrophages, and microglia by amphotericin B and macrophage colony-stimulating factor promotes remyelination. J. Neurosci. 35, 1136–1148 (2015).
Natrajan, M. S., et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain 2, 1071–1084 (2015).
Frenkel, D. et al. Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat. Commun. 4, 2030 (2013).
Fawcett, J. W. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog. Brain. Res. 218, 213–226 (2015).
Orr, M. B. & Gensel, J. C. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15, 541–553 (2018).
Biber, K., Moller, T., Boddeke, E. & Prinz, M. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat. Rev. Drug Discov. 15, 110–124 (2016).
Zhang, C. et al. Cromolyn reduces levels of the Alzheimer’s disease-associated amyloid beta-protein by promoting microglial phagocytosis. Sci. Rep. 8, 1144 (2018).
Rawji, K. S. et al. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain 139, 653–661 (2016).
DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: the devil is in the details. J. Neurochem. 139(Suppl 2), 136–153 (2016).
Acknowledgements
The authors acknowledge operating grant support from the Canadian Institutes of Health Sciences and the Multiple Sclerosis Society of Canada (to V.W.Y.) and from the National Natural Science Foundation of China (grants no: 81870942, 81471174, and 81520108011) and Innovation Scientists and Technicians Troop Constructions Projects of Henan Province of China (for M.X.).
Author information
Authors and Affiliations
Contributions
All coauthors provided sections of the first draft, with the majority being contributed by H.Y.F.Y. and K.S.R. All authors edited the manuscript, and V.W.Y. approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Yong, H.Y.F., Rawji, K.S., Ghorbani, S. et al. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol 16, 540–546 (2019). https://doi.org/10.1038/s41423-019-0223-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0223-3
This article is cited by
-
Hypoxia inducible factor-1α regulates microglial innate immune memory and the pathology of Parkinson’s disease
Journal of Neuroinflammation (2024)
-
Degeneracy in epilepsy: multiple routes to hyperexcitable brain circuits and their repair
Communications Biology (2023)
-
Crosstalk Between Matrix Metalloproteinases and Their Inducer EMMPRIN/CD147: a Promising Therapeutic Target for Intracerebral Hemorrhage
Translational Stroke Research (2023)
-
The macrophage: a key player in the pathophysiology of peripheral neuropathies
Journal of Neuroinflammation (2022)
-
APOE in the bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases
Molecular Neurodegeneration (2022)