Abstract
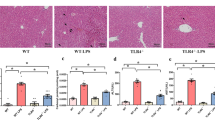
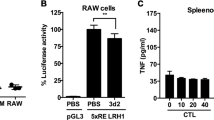
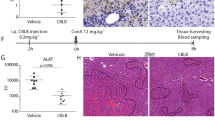
Classical signaling lymphocyte activating molecule (SLAM) family receptors are abundant within many types of immune cells, whereas the nonclassical SLAM family receptors SLAMF8 and SLAMF9, which uniquely lack cytoplasmic signaling motifs, are highly expressed by myeloid cells. Due to the potential redundancy, whether these two receptors regulate macrophage function remains largely unknown. Here, we show that SLAMF8 and SLAMF9 co-regulate macrophage-mediated liver inflammation. To overcome the redundancy, we generated mice that simultaneously lacked SLAMF8 and SLAMF9 using CRISPR-Cas9 technology. Although macrophage differentiation was not altered by the combined deficiency of SLAMF8 and SLAMF9, the loss of these two receptors significantly protected against lipopolysaccharide (LPS)-induced liver injury. SLAMF8 and SLAMF9 double-deficient mice had a prolonged survival rate and less infiltration of inflammatory cells. The depletion of macrophages using clodronate liposomes abolished the effects of SLAMF8 and SLAMF9 deficiencies on LPS-induced liver injury, which demonstrates that these receptors are required for macrophage activation following LPS challenge. Moreover, the deficiency of SLAMF8 and SLAMF9 suppressed the secretion of inflammatory cytokines by downregulating the expression of Toll-like receptor-4 (TLR4), a receptor that specifically binds LPS, which led to decreased mitogen-activated protein kinases (MAPK) signaling activation. Notably, combined injections of truncated extracellular SLAMF8 and SLAMF9 proteins significantly alleviated LPS-induced liver injury. Thus, our findings provide insights into the role of SLAMF8 and SLAMF9 in endotoxin-induced liver injury and suggest that SLAMF8 and SLAMF9 are potential therapeutic targets for acute hepatic injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bernal, W., Auzinger, G., Dhawan, A. & Wendon, J. Acute liver failure. Lancet 376, 190–201 (2010).
Sun, S. et al. Complement and the alternative pathway play an important role in LPS/D-GalN-induced fulminant hepatic failure. PLoS One 6, e26838 (2011).
Schmocker, C. et al. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology 45, 864–869 (2007).
Ma, K., Zhang, Y., Zhu, D. & Lou, Y. Protective effects of asiatic acid against d-galacto-samine/lipopolysaccharide-induced hepatotoxicity in hepatocytes and kupffer cells co-cultured system via redox-regulated leukotriene C4 synthase expression pathway. Eur. J. Pharmacol. 603, 98–107 (2009).
Tsutsui, H. & Nishiguchi, S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int. J. Mol. Sci. 15, 7711–7730 (2014).
Galanos, C., Freudenberg, M. A. & Reutter, W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl Acad. Sci. USA 76, 5939–5943 (1979).
Xu, F. L. et al. Glycine attenuates endotoxin-induced liver injury by downregulating TLR4 signaling in Kupffer cells. Am. J. Surg. 196, 139–148 (2008).
Bedard, K. & Krause, K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007).
Chi, H. et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl Acad. Sci. USA 103, 2274–2279 (2006).
Hoshino, K. et al. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162, 3749–3752 (1999).
Calpe, S. et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv. Immunol. 97, 177–250 (2008).
Van Driel, B. J., Liao, G., Engel, P. & Terhorst, C. Responses to microbial challenges by SLAMF receptors. Front. Immunol. 7, 4 (2016).
De Calisto, J. et al. SAP-dependent and -independent regulation of innate T cell development involving SLAMF receptors. Front. Immunol. 5, 186 (2014).
Chen, S. et al. The self-specific activation receptor SLAM family is critical for NK cell education. Immunity 45, 292–304 (2016).
Chen, S. et al. Dissection of SAP-dependent and SAP-independent SLAM family signaling in NKT cell development and humoral immunity. J. Exp. Med. 214, 475–489 (2017).
Cannons, J. L., Tangye, S. G. & Schwartzberg, P. L. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 29, 665–705 (2011).
Wu, C. B. et al. Genomic organization and characterization of mouse SAP, the gene that is altered in X-linked lymphoproliferative disease. Immunogenetics 51, 805–815 (2000).
Fennelly, J. A., Tiwari, B., Davis, S. J. & Evans, E. J. CD2F-10: a new member of the CD2 subset of the immunoglobulin superfamily. Immunogenetics 53, 599–602 (2001).
Lara-Astiaso, D. et al. Immunogenetics. Chromatin state dynamics during blood formation. Science 345, 943–949 (2014).
Wang, G. et al. Cutting edge: Slamf8 is a negative regulator of Nox2 activity in macrophages. J. Immunol. 188, 5829–5832 (2012).
Dong, Z. et al. The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity 36, 974–985 (2012).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Wang, W. et al. Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-kappa B pathways. Sci. Rep. 7, 44252 (2017).
Wang, G. X. et al. Migration of myeloid cells during inflammation is differentially regulated by the cell surface receptors Slamf1 and Slamf8. PLoS One 10, e0121968 (2015).
Rajaiah, R., Perkins, D. J., Ireland, D. D. & Vogel, S. N. CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance. Proc. Natl Acad. Sci. USA 112, 8391–8396 (2015).
Yang, Z. et al. Differential role for p120-catenin in regulation of TLR4 signaling in macrophages. J. Immunol. 193, 1931–1941 (2014).
Ghosh, M., Subramani, J., Rahman, M. M. & Shapiro, L. H. CD13 restricts TLR4 endocytic signal transduction in inflammation. J. Immunol. 194, 4466–4476 (2015).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81725007, 81771666, and 81471523), Natural Science Foundation of Beijing Municipality (5172018) and 111 Project (B16201).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zeng, X., Liu, G., Peng, W. et al. Combined deficiency of SLAMF8 and SLAMF9 prevents endotoxin-induced liver inflammation by downregulating TLR4 expression on macrophages. Cell Mol Immunol 17, 153–162 (2020). https://doi.org/10.1038/s41423-018-0191-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-018-0191-z
Keywords
This article is cited by
-
The relationship between extreme inter-individual variation in macrophage gene expression and genetic susceptibility to inflammatory bowel disease
Human Genetics (2024)
-
Single-cell transcriptomic landscapes of a rare human laryngeal chondrosarcoma
Journal of Cancer Research and Clinical Oncology (2022)
-
Evidence for the loss and recovery of SLAMF9 during human evolution: implications on Dollo’s law
Immunogenetics (2021)