Abstract
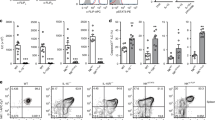
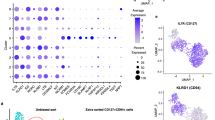
Helper-type innate lymphoid cells (ILC) play an important role in intestinal homeostasis. Members of the NKR-P1 gene family are expressed in various innate immune cells, including natural killer (NK) cells, and their cognate Clr ligand family members are expressed in various specialized tissues, including the intestinal epithelium, where they may play an important role in mucosal-associated innate immune responses. In this study, we show that the inhibitory NKR-P1B receptor, but not the Ly49 receptor, is expressed in gut-resident NK cells, ILC, and a subset of γδT cells in a tissue-specific manner. ILC3 cells constitute the predominant cell subset expressing NKR-P1B in the gut lamina propria. The known NKR-P1B ligand Clr-b is broadly expressed in gut-associated cells of hematopoietic origin. The genetic deletion of NKR-P1B results in a higher frequency and number of ILC3 and γδT cells in the gut lamina propria. However, the function of gut-resident ILC3, NK, and γδT cells in NKR-P1B-deficient mice is impaired during gastrointestinal tract infection by Citrobacter rodentium or Salmonella typhimurium, resulting in increased systemic bacterial dissemination in NKR-P1B-deficient mice. Our findings highlight the role of the NKR-P1B:Clr-b recognition system in the modulation of intestinal innate immune cell functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Leach, M. W., Bean, A. G., Mauze, S., Coffman, R. L. & Powrie, F. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+T cells. Am. J. Pathol. 148, 1503–1515 (1996).
Pene, J. et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J. Immunol. 180, 7423–7430 (2008).
Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8, 411–420 (2008).
Spits, H. & Di Santo, J. P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12, 21–27 (2011).
Eberl, G. et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Hwang, Y. Y. & McKenzie, A. N. Innate lymphoid cells in immunity and disease. Adv. Exp. Med. Biol. 785, 9–26 (2013).
Yang, H., Antony, P. A., Wildhaber, B. E. & Teitelbaum, D. H. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J. Immunol. 172, 4151–4158 (2004).
Inagaki-Ohara, K. et al. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J. Immunol. 173, 1390–1398 (2004).
Halary, F. et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 201, 1567–1578 (2005).
Yokoyama, W. M. & Plougastel, B. F. Immune functions encoded by the natural killer gene complex. Nat. Rev. Immunol. 3, 304–316 (2003).
Carlyle, J. R. et al. Evolution of the Ly49 and Nkrp1 recognition systems. Semin. Immunol. 20, 321–330 (2008).
Belanger, S. et al. Impaired natural killer cell self-education and “missing-self” responses in Ly49-deficient mice. Blood 120, 592–602 (2012).
Rahim, M. M. A. et al. The mouse NKR-P1B:Clr-b recognition system is a negative regulator of innate immune responses. Blood 125, 2217–2227 (2015).
Aldemir, H. et al. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J. Immunol. 175, 7791–7795 (2005).
Rosen, D. B. et al. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J. Immunol. 175, 7796–7799 (2005).
Lanier, L. L., Chang, C. & Phillips, J. H. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J. Immunol. 153, 2417–2428 (1994).
Takahashi, T., Dejbakhsh-Jones, S. & Strober, S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J. Immunol. 176, 211–216 (2006).
Iiai, T. et al. CD161+T (NT) cells exist predominantly in human intestinal epithelium as well as in liver. Clin. Exp. Immunol. 129, 92–98 (2002).
Maggi, L. et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur. J. Immunol. 40, 2174–2181 (2010).
Pesenacker, A. M. et al. CD161 defines the subset of FoxP3+T cells capable of producing proinflammatory cytokines. Blood 121, 2647–2658 (2013).
Mjosberg, J. M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 12, 1055–1062 (2011).
Crellin, N. K., Trifari, S., Kaplan, C. D., Cupedo, T. & Spits, H. Human NKp44+IL-22+cells and LTi-like cells constitute a stable RORC+lineage distinct from conventional natural killer cells. J. Exp. Med. 207, 281–290 (2010).
Allan, D. S. et al. An in vitro model of innate lymphoid cell function and differentiation. Mucosal Immunol. 8, 340–351 (2015).
Leibelt, S. et al. Dedicated immunosensing of the mouse intestinal epithelium facilitated by a pair of genetically coupled lectin-like receptors. Mucosal Immunol. 8, 232–242 (2015).
Zhang, Q. et al. Mouse Nkrp1-Clr gene cluster sequence and expression analyses reveal conservation of tissue-specific MHC-independent immunosurveillance. PLoS ONE 7, e50561 (2012).
Rutkowski, E. et al. Clr-a: a novel immune-related C-type lectin-like molecule exclusively expressed by mouse gut epithelium. J. Immunol. 198, 916–926 (2017).
Kartsogiannis, V. et al. Osteoclast inhibitory lectin, an immune cell product that is required for normal bone physiology in vivo. J. Biol. Chem. 283, 30850–30860 (2008).
Carlyle, J. R. et al. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc. Natl Acad. Sci. USA 101, 3527–3532 (2004).
Mortha, A. et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288 (2014).
Kim, S. H., Cho, B. H., Kiyono, H. & Jang, Y. S. Microbiota-derived butyrate suppresses group 3 innate lymphoid cells in terminal ileal Peyer’s patches. Sci. Rep. 7, 3980 (2017).
Gury-BenAri, M. et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 166, 1231–1246 (2016).
Chen, P. et al. Genetic investigation of MHC-independent missing-self recognition by mouse NK cells using an in vivo bone marrow transplantation Model. J. Immunol. 194, 2909–2918 (2015).
Luci, C. & Reynders, A. & Ivanov, II & Cognet, C. & Chiche, L. & Chasson, L. et al. Influence of the transcription factor RORgammat on the development of NKp46+cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Sanos, S. L. et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+cells. Nat. Immunol. 10, 83–91 (2009).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Ashkar, A. A., Reid, S., Verdu, E. F., Zhang, K. & Coombes, B. K. Interleukin-15 and NK1.1+ cells provide innate protection against acute Salmonella enterica serovar Typhimurium infection in the gut and in systemic tissues. Infect. Immun. 77, 214–222 (2009).
Sonnenberg, G. F., Monticelli, L. A., Elloso, M. M., Fouser, L. A. & Artis, D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134 (2011).
Kupz, A. et al. Contribution of Thy1+NK cells to protective IFN-gamma production during Salmonella typhimurium infections. Proc. Natl Acad. Sci. USA 110, 2252–2257 (2013).
Dolowschiak, T. et al. IFN-gamma hinders recovery from mucosal inflammation during antibiotic therapy for Salmonella gut infection. Cell Host Microbe 20, 238–249 (2016).
Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Inngjerdingen, M., Kveberg, L. & Vaage, J. T. A novel NKR-P1B(bright) NK cell subset expresses an activated CD25(+)CX(3)CR1(+)CD62L(−)CD11b(−)CD27(−) phenotype and is prevalent in blood, liver, and gut-associated lymphoid organs of rats. J. Immunol. 188, 2499–2508 (2012).
Ganal, S. C. et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 37, 171–186 (2012).
Buonocore, S., Ahern, P. P., Uhlig, H. H., Ivanov, I. I., Littman, D. R. & Maloy, K. J. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Sonnenberg, G. F., Fouser, L. A. & Artis, D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390 (2011).
Hoshina, T., Kusuhara, K., Saito, M., Mizuno, Y. & Hara, T. NKRP1A+gammadelta and alphabeta T cells are preferentially induced in patients with Salmonella infection. Hum. Immunol. 73, 623–628 (2012).
Joncker, N. T., Fernandez, N. C., Treiner, E., Vivier, E. & Raulet, D. H. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J. Immunol. 182, 4572–4580 (2009).
Tai, L. H. et al. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J. Exp. Med. 205, 3187–3199 (2008).
Satoh-Takayama, N. et al. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity 41, 776–788 (2014).
Seo, S. U. et al. Intestinal macrophages arising from CCR2(+) monocytes control pathogen infection by activating innate lymphoid cells. Nat. Commun. 6, 8010 (2015).
Aguilar, O. A. et al. A viral immunoevasin controls innate immunity by targeting the prototypical natural killer cell receptor family. Cell 169, 58–71 (2017).
Rahim, M. M. et al. Expansion and protection by a virus-specific NK cell subset lacking expression of the inhibitory NKR-P1B receptor during murine cytomegalovirus infection. J. Immunol. 197, 2325–2337 (2016).
Onyeagocha, C. et al. Latent cytomegalovirus infection exacerbates experimental colitis. Am. J. Pathol. 175, 2034–2042 (2009).
Acknowledgements
We thank Drs. Vicky Kartsogiannis and Matthew T. Gillespie (Monash Medical Centre, Clayton, VIC, Australia) for providing the Clr-b-deficient (Ocil–/–) mice, Dr. Koho Iizuka (University of Minnesota, Minneapolis, MN, USA) for providing the anti-NKR-P1B (2D12) hybridoma, and Dr. Subash Sad (University of Ottawa, Ottawa, Canada) for providing S. typhimurium bacterium. We further thank Drs. Philpott and Banks (University of Toronto) at the germ-free core facility for providing germ-free animals. This work was supported by Operating Grants from the Canadian Institutes of Health Research (CIHR 86630 to A.P.M. and J.R.C. and CIHR 388337 to A.M.).
Author information
Authors and Affiliations
Contributions
E.A.-S., Z.H., J.F., O.A.A., M.S., A.B.M., M.M.T., S.P., A.M., and M.M.A.R. performed the experiments and analyzed the data. E.A.-S., J.R.C., A.M., M.M.A.R., and A.P.M. designed the experiments, analyzed the data, and wrote the manuscript. A.P.M. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Abou-Samra, E., Hickey, Z., Aguilar, O.A. et al. NKR-P1B expression in gut-associated innate lymphoid cells is required for the control of gastrointestinal tract infections. Cell Mol Immunol 16, 868–877 (2019). https://doi.org/10.1038/s41423-018-0169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-018-0169-x
Key words
This article is cited by
-
Clr-f expression regulates kidney immune and metabolic homeostasis
Scientific Reports (2022)
-
Alveolar macrophage metabolic programming via a C-type lectin receptor protects against lipo-toxicity and cell death
Nature Communications (2022)
-
The pathogenic role of innate lymphoid cells in autoimmune-related and inflammatory skin diseases
Cellular & Molecular Immunology (2020)