Abstract
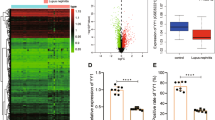
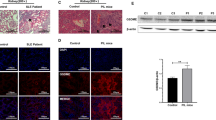
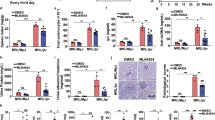
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease, and the pathogenesis of SLE has not been fully elucidated. The E3 ubiquitin ligase FBXW7 has been well characterized in cancer as a tumor suppressor that can promote the ubiquitination and subsequent degradation of various oncoproteins; however, the potential role of FBXW7 in autoimmune diseases is unclear. In the present study, we identified that FBXW7 is a crucial exacerbating factor for SLE development and progression in a mouse model induced by 2, 6, 10, 14-tetramethylpentadecane (TMPD). Myeloid cell-specific FBXW7-deficient (Lysm+FBXW7f/f) C57BL/6 mice showed decreased immune complex accumulation, glomerulonephritis, glomerular mesangial cell proliferation, and base-membrane thickness in the kidney. Lysm+FBXW7f/f mice produced fewer anti-Sm/RNP and anti-ANA autoantibodies and showed a decreased MHC II expression in B cells. In Lysm+FBXW7f/f mice, we observed that cell apoptosis was reduced and that fewer CD11b+Ly6Chi inflammatory monocytes were recruited to the peritoneal cavity. Consistently, diffuse pulmonary hemorrhage (DPH) was also decreased in Lysm+FBXW7f/f mice. Mechanistically, we clarified that FBXW7 promoted TMPD-induced cell apoptosis by catalyzing MCL1 degradation through K48-linked ubiquitination. Our work revealed that FBXW7 expression in myeloid cells played a crucial role in TMPD-induced SLE progression in mice, which may provide novel ideas and theoretical support for understanding the pathogenesis of SLE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rahman, A. & Isenberg, D. A. Systemic lupus erythematosus. N. Engl. J. Med. 358, 929–939 (2008).
Lech, M. & Anders, H. J. The pathogenesis of lupus nephritis. J. Am. Soc. Nephrol. 24, 1357–1366 (2013).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Moulton, V. R. et al. Pathogenesis of human systemic lupus erythematosus: a cellular perspective. Trends Mol. Med. 23, 615–635 (2017).
Mahajan, A., Herrmann, M. & Munoz, L. E. Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front. Immunol. 7, 35 (2016).
Fenton, K. The effect of cell death in the initiation of lupus nephritis. Clin. Exp. Immunol. 179, 11–16 (2015).
Mistry, P. & Kaplan, M. J. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin. Immunol. 185, 59–73 (2017).
Sharma, S., Fitzgerald, K. A., Cancro, M. P. & Marshak-Rothstein, A. Nucleic acid-sensing receptors: rheostats of autoimmunity and autoinflammation. J. Immunol. 195, 3507–3512 (2015).
Kirou, K. A. et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Jonsson, C. A., Erlandsson, M., Svensson, L., Molne, J. & Carlsten, H. Mycophenolate mofetil ameliorates perivascular T lymphocyte inflammation and reduces the double-negative T cell population in SLE-prone MRLlpr/lpr mice. Cell Immunol. 197, 136–144 (1999).
Mannoor, M. K. et al. Honeybee royal jelly inhibits autoimmunity in SLE-prone NZB x NZW F1 mice. Lupus 18, 44–52 (2009).
Lewis, R. M. et al. Chronic allogeneic disease. I. Development of glomerulonephritis. J. Exp. Med. 128, 653–679 (1968).
Satoh, M. et al. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin. Exp. Immunol. 121, 399–405 (2000).
Wang, Z. et al. Beneficial effect of Bupleurum polysaccharides on autoimmune disease induced by Campylobacter jejuni in BALB/c mice. J. Ethnopharmacol. 124, 481–487 (2009).
Lee, P. Y. et al. A novel type I IFN-producing cell subset in murine lupus. J. Immunol. 180, 5101–5108 (2008).
Bossaller, L. et al. TLR9 deficiency leads to accelerated renal disease and myeloid lineage abnormalities in pristane-induced murine lupus. J. Immunol. 197, 1044–1053 (2016).
Reeves, W. H., Lee, P. Y., Weinstein, J. S., Satoh, M. & Lu, L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 30, 455–464 (2009).
Huang, W. et al. Milk fat globule-EGF factor 8 suppresses the aberrant immune response of systemic lupus erythematosus-derived neutrophils and associated tissue damage. Cell Death Differ. 24, 263–275 (2017).
Calvani, N. et al. Induction of apoptosis by the hydrocarbon oil pristane: implications for pristane-induced lupus. J. Immunol. 175, 4777–4782 (2005).
Jiang, X. & Chen, Z. J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 12, 35–48 (2012).
Wang, Z. et al. Tumor suppressor functions of FBW7 in cancer development and progression. FEBS Lett. 586, 1409–1418 (2012).
Bednash, J. S. & Mallampalli, R. K. Regulation of inflammasomes by ubiquitination. Cell Mol Immunol. 13, 722–728 (2016).
Li, J., Chai, Q. Y. & Liu, C. H. The ubiquitin system: a critical regulator of innate immunity and pathogen-host interactions. Cell. Mol. Immunol. 13, 560–576 (2016).
Pickart, C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001).
Shimizu, Y., Taraborrelli, L. & Walczak, H. Linear ubiquitination in immunity. Immunol. Rev. 266, 190–207 (2015).
Chen, Z. J. & Sun, L. J. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 33, 275–286 (2009).
Skaar, J. R., Pagan, J. K. & Pagano, M. SCF ubiquitin ligase-targeted therapies. Nat. Rev. Drug Discov. 13, 889–903 (2014).
Xie, C. M., Wei, W. & Sun, Y. Role of SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases in skin cancer. J. Genet. Genomics 40, 97–106 (2013).
Welcker, M. et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl Acad. Sci. USA 101, 9085–9090 (2004).
Popov, N., Herold, S., Llamazares, M., Schulein, C. & Eilers, M. Fbw7 and Usp28 regulate myc protein stability in response to DNA damage. Cell Cycle 6, 2327–2331 (2007).
Strohmaier, H. et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413, 316–322 (2001).
Koepp, D. M. et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 (2001).
O’Neil, J. et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J. Exp. Med. 204, 1813–1824 (2007).
Balamurugan, K. et al. FBXW7alpha attenuates inflammatory signalling by downregulating C/EBPdelta and its target gene Tlr4. Nat. Commun. 4, 1662 (2013).
Song, Y. et al. E3 ligase FBXW7 is critical for RIG-I stabilization during antiviral responses. Nat. Commun. 8, 14654 (2017).
Ferenbach, D. A. & Bonventre, J. V. Kidney tubules: intertubular, vascular, and glomerular cross-talk. Curr. Opin. Nephrol. Hypertens. 25, 194–202 (2016).
Nowling, T. K. & Gilkeson, G. S. Mechanisms of tissue injury in lupus nephritis. Arthritis Res. Ther. 13, 250 (2011).
Mak, A. et al. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin. Arthritis Rheum. 41, 830–839 (2012).
Thomas, G. et al. Mortality associated with systemic lupus erythematosus in France assessed by multiple-cause-of-death analysis. Arthritis Rheumatol. 66, 2503–2511 (2014).
Xu, Y. et al. Pleiotropic IFN-dependent and -independent effects of IRF5 on the pathogenesis of experimental lupus. J. Immunol. 188, 4113–4121 (2012).
Barrat, F. J., Elkon, K. B. & Fitzgerald, K. A. Importance of nucleic acid recognition in inflammation and autoimmunity. Annu. Rev. Med. 67, 323–336 (2016).
Lee, P. Y. et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J. Exp. Med. 205, 2995–3006 (2008).
Bossaller, L. et al. Overexpression of membrane-bound fas ligand (CD95L) exacerbates autoimmune disease and renal pathology in pristane-induced lupus. J. Immunol. 191, 2104–2114 (2013).
Zamora, M. R., Warner, M. L., Tuder, R. & Schwarz, M. I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine 76, 192–202 (1997).
Shi, Y. et al. Pristane-induced granulocyte recruitment promotes phenotypic conversion of macrophages and protects against diffuse pulmonary hemorrhage in Mac-1 deficiency. J. Immunol. 193, 5129–5139 (2014).
Primack, S. L., Miller, R. R. & Muller, N. L. Diffuse pulmonary hemorrhage: clinical, pathologic, and imaging features. Am. J. Roentgenol. 164, 295–300 (1995).
Janz, S. & Shacter, E. A new method for delivering alkanes to mammalian cells: preparation and preliminary characterization of an inclusion complex between beta-cyclodextrin and pristane (2,6,10,14-tetramethylpentadecane). Toxicology 69, 301–315 (1991).
Davis, R. J., Welcker, M. & Clurman, B. E. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell 26, 455–464 (2014).
Pawlikowska, P. et al. ATM-dependent expression of IEX-1 controls nuclear accumulation of Mcl-1 and the DNA damage response. Cell Death Differ. 17, 1739–1750 (2010).
Inuzuka, H. et al. Mcl-1 ubiquitination and destruction. Oncotarget 2, 239–244 (2011).
Inuzuka, H. et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471, 104–109 (2011).
Wertz, I. E. et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471, 110–114 (2011).
Kwon, Y. T. & Ciechanover, A. The ubiquitin code in the ubiquitin-proteasome system and autophagy. Trends Biochem. Sci. 42, 873–886 (2017).
Denny, M. F. et al. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J. Immunol. 176, 2095–2104 (2006).
Mevorach, D., Zhou, J. L., Song, X. & Elkon, K. B. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 188, 387–392 (1998).
Cohen, S. Diffuse pulmonary hemorrhage: evolutionary ‘flaw’ or consequence of evolutionary progress? Am. J. Med. Sci. 323, 130–139 (2002).
Murphy, M. P. & Caraher, E. Mcl-1 is vital for neutrophil survival. Immunol. Res. 62, 225–233 (2015).
Wang, F. Y. et al. Suppression of Mcl-1 induces apoptosis in mouse peritoneal macrophages infected with Mycobacterium tuberculosis. Microbiol. Immunol. 60, 215–227 (2016).
Acknowledgements
We would like to thank Prof. Ximei Wu for providing the Lysm-Cre mice and Prof. Hong Deng for the kidney pathology analysis. This work was supported by the National Natural Science Foundation of China (81771699, 31870907, and 81571524), Natural Science Foundation of Zhejiang Province (Z19H100001), and National Key Basic Research Program of China (2014CB542101).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chong, Z., Bao, C., He, J. et al. E3 ligase FBXW7 aggravates TMPD-induced systemic lupus erythematosus by promoting cell apoptosis. Cell Mol Immunol 15, 1057–1070 (2018). https://doi.org/10.1038/s41423-018-0167-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-018-0167-z