Abstract
Previously, we have identified ZFAS1 as a potential new long non-coding RNA (lncRNA) biomarker of acute myocardial infarction (MI) and as a sarcoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) inhibitor, causing intracellular Ca2+ overload and contractile dysfunction in a mouse model of MI. In the current study, we aimed to evaluate the effects of ZFAS1 on the apoptosis of cardiomyocytes in the MI mouse model. Knockdown of endogenous ZFAS1 by virus-mediated silencing shRNA or siZFAS1 partially abrogated the ischemia-induced apoptosis of cardiomyocytes. Overexpression of ZFAS1 in normal cardiomyocytes reduced the cell viability, similar to that observed in hypoxia-treated cardiomyocytes. Moreover, ZFAS1 cardiac-specific knock-in mice showed impaired cardiac function, adversely altered Ca2+ homeostasis, repressed expression and activities of SERCA2a, and increased apoptosis. At the subcellular level, ZFAS1 induced mitochondrial swelling and showed a pronounced decrease in mitochondrial membrane potential. At the molecular level, ZFAS1 activated the mitochondria apoptosis pathway, which could be nearly abolished by a calcium chelator. The effects of ZFAS1 were readily reversible upon knockdown of this lncRNA. Notably, ZFAS1-FD (only functional domain) mimicked the effects of full-length ZFAS1 in regulation of cardiomyocyte apoptosis. In conclusion, our study shows that ZFAS1, an endogenous SERCA2a inhibitor, induces mitochondria-mediated apoptosis via cytosolic Ca2+ overload. Therefore, anti-ZFAS1 might be considered a new therapeutic strategy for protecting cardiomyocytes from MI-induced apoptosis.
Similar content being viewed by others
Introduction
Myocardial infarction (MI), a leading cause of heart failure, is a severe threat to human lives1,2. The pathogenesis of MI involves multiple conditions, with cardiomyocyte apoptosis being one of the most crucial components3,4. Intrinsic pathway (also called mitochondria-mediated apoptotic pathway) and extrinsic pathway are the two pathways that modulate apoptosis, with the former playing an important role in cardiomyocyte apoptosis5,6. Mitochondria-mediated apoptosis is initiated by intracellular stimuli, such as oxidative stress, hypoxia, and nutrient deprivation, which causes an imbalance in the expression of Bcl2 family proteins (up-regulated pro-apoptotic proteins (Bax) and downregulated anti-apoptotic proteins (Bcl2)), leading to the induction of mitochondrial outer membrane permeabilization (MOMP) and release of cytochrome C. Cytochrome C promotes the assembly of caspase-9-associated apoptosome. Subsequently, caspase-9 triggers the caspase cascade, leading to the activation of caspase-3/7 and finally apoptosis7,8. Recently, studies have confirmed that cytosolic Ca2+ overload could induce mitochondria-mediated apoptosis9,10. Yet, how cytosolic Ca2+ overload inducing cardiomyocytes apoptosis during MI remains poorly understood.
Long noncoding RNAs (lncRNAs), a newly discovered class of non-protein-coding RNAs, have been reported to be involved in multiple heart diseases11,12,13. Growing evidences had demonstrated that lncRNA-ZFAS1, an antisense lncRNA to the 5′ end of the protein-coding gene Znfx1, had a significant role in the regulation of tumor14,15,16. In our previous study, we identified ZFAS1 as an independent predictor of acute MI (AMI)17. Additionally, we found that ZFAS1 bound to and inhibited the intracellular level and activity of sarcoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) protein, and contributed to the impairment of cardiac contractile function in MI18. Therefore, ZFAS1 might be considered as a new therapeutic target for preserving cardiac function under pathological conditions of the heart. However, it is not yet clear whether ZFAS1 is involved in mitochondria-mediated cardiomyocyte apoptosis via cytosolic Ca2+ overload.
In the current study, we aimed to further clarify the role of ZFAS1 in mitochondria-mediated cardiomyocyte apoptosis by loss and gain of function approaches in MI mice model. The results of this study are expected to provide a basis for developing novel therapeutic strategies for protecting cardiomyocytes from MI-induced apoptosis.
Materials and methods
The mouse model of MI
A mouse model of MI was obtained by left anterior descending coronary artery (LAD) occlusion with C57BL/6 mice ranging from 8 to 10 weeks in age and weighing between 22 g and 25 g as described previously in detail19. Significant elevation of S-T segment in electrocardiograph (ECG) was observed in the MI group. The mice were sacrificed at 12 h after MI. The sacrificed mice were removed and randomization and blinding were adopted in animal experiments. Use of animals was approved by the Ethic Committees of Harbin Medical University.
Cardiac-specific ZFAS1 knock-in mice
Cardiac-specific ZFAS1 knock-in (TG) mice were generated by crossing ZFAS1 flox/flox mice (Cyagen Biosciences Inc.) with C57BL/6 background and a-myosin heavy chain promoter–driven Cre mice (αMHC-Cre, Cyagen Biosciences Inc.) as described previously20.
Echocardiographic assessment of cardiac function
The left ventricular internal dimension at end-diastole (LVIDd), left ventricular internal dimension at systole (LVIDs), and ejection fraction (EF) of mice models were assessed by an echocardiographic system (Visualsonics, Toronto, ON, Canada) as described previously21. The fractional shortening (FS) was calculated according to the equation: (LVIDd−LVIDs)/LVIDd × 100.
Construction and delivery of viral vectors for ZFAS1 overexpression and knockdown
AAV9 vectors carrying a short RNA fragment for silencing ZFAS1 (shZFAS1-V, “V” representing virus) or ZFAS1 sequence (ZFAS1-V) were constructed (Lederer biological technology Co., Ltd., Guangzhou, Guangdong, China) as described previously18. C57BL/6 mice received the virus solution (2 × 1011 genome-containing particles (GC)/animal) via tail vein injection. The following experiments would be done after one-time injection of ZFAS1-V or shZFAS1-V for 4 weeks.
Neonatal mouse cardiomyocytes (NMCMs) culture and treatment
Cardiomyocytes isolated from 1 to 3-day-old neonatal mice (C57BL/6) were deprived of serum and placed in an anoxic chamber (5% CO2 and 95% N2) for 12 h to mimic myocardial ischemia in vitro. The ZFAS1-specific siRNA (siZFAS1), commercially synthesized by Ribobio (Guangzhou, Guangdong, China, sense: GCGUGAACUCCUGAGGCGAdTdT, antisense: UCGCCUCAGGAGUUCACGCdTdT) was transfected into cells for ZFAS1 knockdown according to the manufacturer’s protocol. ZFAS1 transcript cDNA, inserted into the pCDNA3.1 (pCDNA-ZFAS1, ZFAS1-P), were constructed and transfected into cells (2 mg/L) for ZFAS1 overexpression. After transfected with pCDNA-ZFAS1 for 24 h, BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) (10 μmol/L) was added for Ca2+ chelation. ZFAS1-FD, synthesized by Lederer Biological Technology (Guangdong, China, 5′-UGCGUGCCAAGCGCGACAUGGCGCGGAAGCCGAGAAGCCCCGGAGGCCC-3′ was transfected into cells for ZFAS1-FD overexpression. Cyclopiazonic acid (CPA) was added to the NMCMs for SERCA2a inhibition at the concentration of 5 μmol/L. The NMCMs were collected for the following experiments after transfection for 48 h or 72 h.
Quantitative real-time PCR (qPCR)
qPCR was performed as previously described22. The data were collected from three separate experiments. Sequences for qPCR primers: ZFAS1 (mouse): forward 5′-AGCGTTTGCTTTGTTCCC-3′ and reverse 5′-CTCCCTCGATGCCCTTCT-3′; SERCA2a (mouse): forward 5′-TAAATGCCCGCTGTTTTGCT-3′ and reverse 5′-TTGTCATCTGCCAGGACCAT-3′; β-actin (mouse): forward 5′-ACTGCCGCATCCTCTTCCT-3′ and reverse 5′-TCAACGTCACACTTCATGATGGA-3′.
MTT assay for cell viability
Cardiomyocytes were cultured in 96-well culture clusters (about 1*104 per well), and then the cells were transfected with ZFAS1-specific siRNA or pCDNA-ZFAS1 plasmid vectors for 48 h. The cells cultured in complete medium under a normoxic atmosphere were considered as blank control. Particularly, some cells need hypoxia treatments. The cells were incubated for 4 h in a medium containing 0.5% 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT). The amount of blue formazan dye formed from MTT is proportional to the number of survival cells. The MTT reaction was terminated by adding DMSO to the medium followed by incubation for 10 min at room temperature. The absorbance was read at 490 nm in a spectrophotometer (BioTek, USA).
TUNEL assay
Terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) staining was used to evaluate the apoptosis of cultured cardiomyocytes. Briefly, cardiac myocytes cultured on coverslips in 24-well plates were fixed in 4% paraformaldehyde. The TUNEL staining was done using the in situ cell death detection kit (Minneapolis, MN, USA) according to the manufacturer’s protocol. The numbers of TUNEL-positive cells and the total cells were counted under a confocal microscopy.
Western blot
Proteins isolated from cells (40–60 μg) or myocardial tissue (80–120 μg) were separated by SDS–PAGE (10–15% polyacrylamide gels). Partitioned proteins were transferred to Pure Nitrocellulose Blotting Membrane (PALL, New York, USA). The membrane was probed with a primary antibodies for SERCA2a (#A1097; ABclonal, Wuhan, China), Caspase3 (Cell Signaling Technology® #9662S), Cleaved Caspase3 (Cell Signaling Technology® #9664S), Caspase9 (Cell Signaling Technology® #9504), Bax (Gene Tex® #GTX32465), Bcl2 (Gene Tex® #GTX100064), p-CAMKII (Affbiotech, #AF3493), CAMKII (Affbiotech, #AF6493), and Connexin43 (EMD Millipore, #AB1727) at 4 °C overnight. Then, the membranes were incubated with secondary antibodies (Jackson Immuno Research, West Grove, PA, USA). Blots were detected and analyzed with the Odyssey v1.2 software (LI-COR Biosciences, Lincoln, NE, USA) for each group and normalized to β-actin or β-Tubulin band intensity. The final results were expressed as fold changes and the experiment was performed in triplicate and repeated three times.
LIVE/DEAD viability/cytotoxicity kit stains
The cultured NMCMs were seeded in six-well plates and the components of the LIVE/DEAD Viability/Cytotoxicity Kit (Cat. L3224, Life technologies, USA) were added to each well. Cells with compromised membranes exhibit red-fluorescence from the live-cell-impermeant nucleic acid stain ethidium homodimer-1. Cells with intact cell membranes could convert nonfluorescent calcein AM into bright green-fluorescent calcein using nonspecific cytosolic esterases. Images were observed by confocal laser microscope (Olympus, Tokyo, Japan).
Assessment of mitochondrial membrane potential
JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide) has been extensively used to study the loss of the mitochondrial membrane potential which occurs during apoptosis. In normal cells, due to high membrane potential, the dye concentrates in the mitochondrial matrix, and it forms red-fluorescent aggregates (J-aggregates). Any event that dissipates the mitochondrial membrane potential prevents the accumulation of the JC-1 dye in the mitochondria and thus, the dye is dispersed throughout the entire cell leading to a shift from red (J-aggregates) to green fluorescence (JC-1 monomers). A decrease in red/green ratio is indicative of apoptosis. The cells were grown in 24-well plate and transfected with ZFAS1-siRNA before hypoxia treating for 12 h. The treated cells were washed with PBS and stained with 2 mg/ml of JC-1 dye in DMEM media at 37 °C in dark for 20 min. Laser confocal microscopy was adopted for observation and photo taking.
Flow cytometry
Apoptosis was detected by measuring phosphatidyl serine exposure and membrane permeability of cells. Cardiomyocytes were harvested and double-stained with FITC-conjugated Annexin V and propidium iodide (PI) (absin Annexin V/FITC Apoptosis Detection Kit). Samples were analyzed by the FACScan flow cytometer with Cell Quest software (Beckman Coulter, USA).
Optical mapping
The real-time changes of intracellular Ca2+ concentration ([Ca2+]i) were measured by optical mapping system (MICAM05, Brainvision, Tokyo, Japan). The optical mapping was performed according to the procedures described previously23. An image-capturing software (BV_MC05E; Brainvision, Tokyo, Japan) was adopted for optical recording of the heart at 6 Hz field stimulation and an image analysis software (BV-Analyze; Brainvision) was used for data analysis.
Measurement of SERCA2a activity
The SERCA2a activity was determined by Ca2+ ATPase assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. Briefly, cardiac tissue or NMCMs were treated as specified. The amount of inorganic phosphate liberated from ATP hydrolysis was used to evaluate the activity of SERCA2a.
Immunofluorescence staining
Immunofluorescence staining was performed on cardiac tissue to determine Cx43 expression. The heart tissue was paraffin-embedded and sectioned for following staining. After dewaxing with xylene and antigen repair, the sectioned tissue was blocked with 10% goat serum overnight and incubated with anti-Cx43 (1:2000), alpha actin (1:300) overnight at 4 °C and then with the conjugated secondary antibody for 1 h. The nuclei were visualized with DAPI (4′,6-diamidino-2-phenylindole) at room temperature for 30 min, and images (×40 magnification) were captured by a confocal fluorescent microscope.
Isolation of adult mouse cardiomyocytes
Cardiomyocytes were isolated from adult ZFAS1 knock-in mice or WT as described previously18. Briefly, adult mice were anesthetized and the hearts were rapidly excised. The aorta of the heart was cannulated on a constant-flow Langendorff apparatus. After digestion, the heart tissue had become softened and gently minced into small chunks, which were then equilibrated in Tyrode’s solution with 200 μM CaCl2 and 1% bovine serum albumin at room temperature.
Data analysis
Pooled data are presented as mean ± SEM values and analyzed with SPSS 13.0 software. Statistical comparisons among multiple groups were performed using analysis of variance (ANOVA) followed by Dunnett’s test. Student's t-test was carried out for comparisons between two groups. A two-tailed P < 0.05 was taken to indicate a statistically significant difference.
Results
Knockdown of ZFAS1 protects cardiomyocytes against MI
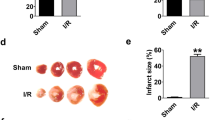
Recently, we demonstrated that ZFAS1 expression is markedly elevated in the myocardium of mice with AMI (12 h post-MI)18. Here, we first established a MI model and confirmed that the cardiac function of the MI mice was decreased obviously (Fig. S1). Furthermore, mitochondria turgescence and fragmented cristae were observed in the heart tissue from MI mice (Fig. 1a). Next, we verified the expression level of ZFAS1 in the cardiac tissue of MI mice and hypoxia-treated NMCMs. Compared with the Sham or Control group, the expression level of ZFAS1 was increased (Fig. S2). We then employed a loss-of-function strategy with adeno-associated virus serotype 9 (AAV9) vector carrying a ZFAS1-shRNA fragment (shZFAS1-V) and investigated if knocking down ZFAS1 could alter pathological conditions of the heart. The efficiency of shZFAS1-V for ZFAS1 knockdown was verified by qRT-PCR (Fig. S3a). In the current study, the mitochondrial swelling and myofibril breakage caused by MI in the cardiac tissue were nearly abolished by shZFAS1-V, as evident under electron microscope (Fig. 1a). In vitro, siZFAS1 increased the viability of NMCMs exposed to hypoxic environment (Fig. 1b). The efficiency of siZFAS1 for ZFAS1 knockdown both in normal and hypoxia-treated NMCMs was verified by qRT-PCR (Fig. S3b, c). Using LIVE/DEAD Viability/Cytotoxicity Kit that stains cells with compromised membranes to show red-fluorescence and cells with intact cell membranes to show bright green-fluorescence, we observed that the hypoxia-treated cardiac myocytes exhibited more number of cells showing red-fluorescence than the control group; however, hypoxia-treated cells transfected with siZFAS1 showed an obvious reduction of red-fluorescence. It indicated that ZFAS1 knockdown could restore the viability of hypoxia-treated cardiomyocytes (Fig. 1c, d). Moreover, the hypoxia treatment induced mitochondria turgescence and fragmented cristae in NMCMs were rescued by ZFAS1 knockdown (Fig. 1e). To further assess the influence of ZFAS1 on mitochondria, the JC-1 assay was done to evaluate the loss of mitochondrial membrane potential, which occurs during apoptosis. The results showed that compared with the control group, the mitochondrial membrane potential of hypoxia-treated NMCMs were notably compromised, presenting as a large area of green fluorescence. However, the siZFAS1-transfected cells exhibited reduced green fluorescence after 12 h of hypoxia treatment compared with the normal control group (Fig. 1f). These results indicated that cells transfected with siZFAS1 might be resistant to hypoxia-induced apoptosis of cardiomyocytes. To clarify this, we investigated the effects of ZFAS1 on apoptosis of cardiomyocytes induced by hypoxia treatment. As shown in Fig. 1g, h, the number of TUNEL-positive cells was significantly decreased after transfection with siZFAS1, whereas siNC did not elicit any significant changes. Apoptosis was determined through PI Annexin V double staining and FACS analysis. As shown in Fig. 1i, j, the percentage of cells stained with Annexin V was increased after hypoxia for 12 h, while it was significantly reduced in case of cells transfected with siZFAS1. These data demonstrated that knockdown of ZFAS1 protects cardiomyocytes against apoptosis.
a Effects of ZFAS1 knockdown on the mitochondrion in the myocardium of MI mice, observed by electron microscope. Yellow arrows pointing to the mitochondrion. Images are presented with a magnification of ×10,000 for the top and ×40,000 for the down panel. It was repeated for three times with similar results. b MTT assay showing that ZFAS1 knockdown increased the cell viability of hypoxia-treated cells. *P < 0.05 vs. Hypoxia + siNC, n = 13. c, d LIVE/DEAD Viability/Cytotoxicity Kit stains were used to detect the effects of ZFAS1 on cardiomyocytes. Cells with compromised membranes exhibit red-fluorescence, while those with intact cell membranes show bright green-fluorescence. *P < 0.05 vs. control group, n = 5; #P < 0.05 vs. hypoxia group, n = 5. Magnification, ×200. e Effects of ZFAS1 knockdown on the mitochondrion in hypoxia-treated cardiomyocytes, visualized by electron microscope (magnification, ×10,000 for the top and ×40,000 for the down panel). Similar results were consistently observed in another two batches of cells. f ZFAS1 knockdown improved the mitochondrial membrane potential, as observed by JC-1 staining. The red-fluorescent aggregates (J-aggregates) represent high membrane potential; green fluorescence (JC-1 monomers) represents dissipated mitochondrial membrane potential. Magnification, ×1200. Similar results were consistently observed in another two batches of cells. g, h The influence on apoptosis with ZFAS1 knockdown was examined via TUNEL assay. Blue, DAPI staining for nucleus; green, TUNEL-positive staining for apoptotic cells. **P < 0.01 vs. control group, #P < 0.05 vs. hypoxia group, the results are expressed as the means ± SEM of four independent experiments. Magnification, ×200. i, j Cells were harvested and processed for apoptosis assay using the Annexin V-FITC Apoptosis Detection Kit by flow cytometry. *P < 0.05 vs. control group, #P < 0.05 vs. hypoxia group, the results are expressed as the means ± SEM of four independent experiments.
Next, we checked the expression of apoptosis-associated proteins from the mitochondria-mediated apoptotic pathway. As shown in Fig. 2a–d, the cardiac expression of caspase-3, caspase-9, and Bax proteins were prominently increased in MI mice and Bcl2 was decreased relative to the sham animals. Notably, shZFAS1-V reversed the abnormal expression of these proteins. Similar patterns of expression alterations of Bax and Bcl2 proteins were consistently observed in NMCMs cultured under hypoxic insult and with treatments with varying constructs (Fig. 2e, f). In all cases, the negative control construct shNC-V and siNC did not affect the deleterious alterations in MI mice and hypoxia-treated NMCMs.
a–d The expression of caspase-3, caspase-9, and Bax was significantly increased, and that of Bcl2 was decreased in heart with myocardial infarction (MI) compared with Sham-operation control mice. The expression was reversed by transfection with AAV9-shZFAS1. *P < 0.05, **P < 0.01 vs. Sham, #P < 0.05 vs. MI, §§P < 0.01 vs. MI + shZFAS1-V; n = 5 for caspase-3, caspase-9, and Bcl2, n = 7 for Bax. e, f The expression of Bax was significantly increased and Bcl2 was decreased in cultured cardiomyocytes after hypoxia treatment for 12 h. Note that silencing ZFAS1 by siZFAS1 normalized the Bax and Bcl2 expression.*P < 0.05 vs. Control, #P < 0.05 vs. Hypoxia; n = 6 for Bax, n = 5 for Bcl2. Data are presented as means ± SEM.
Upregulation of ZFAS1 expression induces cardiomyocyte apoptosis
The data presented above suggested that knockdown of ZFAS1 protects myocardium against MI-induced cardiomyocytes apoptosis. If this is true, overexpression of ZFAS1 in otherwise normal mice and NMCMs should be able to reproduce the phenotypes of MI and hypoxia-induced apoptosis. To test this hypothesis, we utilized the gain-of-function approach for our subsequent experiments. The AAV9 vector carrying the ZFAS1 gene (ZFAS1-V) was constructed for its overexpression under in vivo conditions and the pCDNA-ZFAS1 vector (ZFAS1-P) was transfected to NMCMs for overexpression in vitro conditions. The efficiency of ZFAS1-V and ZFAS1-P for ZFAS1 overexpression were verified by qRT-PCR (Fig. S4). In sharp contrast to ZFAS1 silencing, forced expression of ZFAS1 with ZFAS1-V by tail vein injection caused mitochondrial swelling, as observed under electron microscope (Fig. 3a), and elevated expression of caspase-3, caspase-9, and Bax, and decreased expression of Bcl2 at the protein level (Fig. 3b–e). At the cellular level, ZFAS1-P-transfected NMCMs exhibited impaired cell viability (Fig. 3f–h). Moreover, TUNEL assay demonstrated that ZFAS1 overexpression caused cardiomyocyte apoptosis (Fig. 3i, j). At the subcellular level, ZFAS1-P-transfected NMCMs showed downregulated Bcl2 protein and upregulated expression of Bax protein (Fig. 3k, l).
a Electron microscopy images showing the effects of ZFAS1 overexpression on the mitochondria in the myocardium of mice (magnification, ×10,000 for the top and ×40,000 for the down panel). b–e The expression of apoptosis-related proteins including caspase-3, caspase-9, Bcl2, and Bax were quantified by western blot. *P < 0.05, **P < 0.01 vs. control group, n = 6 for caspase-3 and caspase-9, n = 5 for Bcl2 and Bax. f MTT assay to evaluate the cell viability of ZFAS1-overexpressing cardiacmyocytes generated by pCDNA-ZFAS1 vector (ZFAS1-P) transfection in nonhypoxia cardiomyocytes. **P < 0.01 vs. Control Group, n = 13. g, h LIVE/DEAD Viability/Cytotoxicity Kit stains were used to detect the effect of ZFAS1 overexpression on the viability of cardiacmyocytes. *P < 0.05 vs. Control Group, n = 7. Magnification, ×200. i, j The effect of ZFAS1 on cardiomyocyte apoptosis was validated by TUNEL assay. Blue, DAPI staining for nucleus; green, TUNEL-positive staining for apoptotic cells. *P < 0.05 vs. control group, the results are expressed as the means ± SEM of four independent experiments. Magnification, ×200. k, l The expression of Bcl2 was significantly decreased and Bax was increased in cultured cardiomyocytes after ZFAS1 overexpression. *P < 0.05 vs. control group, n = 5. Data are presented as means ± SEM.
Downregulation and dysfunction of SERCA2a induce cardiomyocyte apoptosis in ZFAS1 transgenic mice (TG)
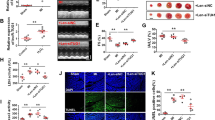
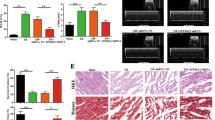
To further investigate the role of ZFAS1 in MI, ZFAS1 cardiac-specific knock-in (TG) mice were constructed (Fig. 4a). The expression of ZFAS1 in the cardiac tissue of the TG was verified by qRT-PCR (Fig. 4b). As depicted in Fig. 4c, TG showed significantly reduced EF and FS, and enlarged LVIDd and LVIDs. Similar to our previous study, ZFAS1 knock-in mice exhibited decreased expression of SERCA2a both at the protein and mRNA levels (Fig. 4d, Fig. S5). Additionally, TG showed impaired activity of SERCA2a in the cardiac tissue (Fig. 4e). Moreover, we evaluated the effects of ZFAS1 on the expression of other proteins that can modify SERCA activity or regulate cardiomyocyte–cardiomyocyte coupling. As shown in Fig. S6, ZFAS1 had no obvious effects on the expression of P-CaMKII, CaMKII, or Cx43 protein levels. The calcium homeostasis of the TG was also assessed by optical-mapping techniques. Intriguingly, as depicted in Fig. 4f, the decaying phase time due to Ca2+ was significantly delayed in the TG compared with the wide type mice (WT). The resting intracellular Ca2+ in the cardiomyocytes isolated from TG was significantly increased comparing with WT (Fig. S7). Additionally, compared with the WT group, the expression of apoptosis-associated proteins—caspase-9, caspase-3, cleaved caspase-3—were significantly increased and Bcl2 was decreased in the cardiac tissue of TG (Fig. 4g–j). Collectively, downregulation and dysfunction of SERCA2a induced cardiomyocytes apoptosis in ZFAS1-TG.
a Schematic diagram of the generation of cardiac-specific ZFAS1 knock-in mice. b Verification of the expression of ZFAS1 in the heart tissue of ZFAS1 knock-in mice (TG).**P < 0.01 vs. wide type (WT), n = 14. c The cardiac function of ZFAS1 TG mice. ZFAS1 TG mice showed decreased ejection fraction (EF) and fractional shortening (FS), and enlarged left ventricular internal dimension at end-diastole (LVIDd) and left ventricular internal dimension at systole (LVIDs).**P < 0.01 vs. WT, n = 12. d Downregulation of SERCA2a expression in ZFAS1 knock-in mice at protein levels.*P < 0.05 vs. WT, n = 7. e Downregulation of SERCA2a activity in ZFAS1 knock-in mice.**P < 0.01 vs. WT, n = 5. f Impairment of intracellular Ca2+ homeostasis by ZFAS1, assessed by optical-mapping techniques in ZFAS1 knock-in mice. g–j Western-blot analysis of the expression of apoptosis-related proteins in TG mice.*P < 0.05,**P < 0.01 vs. WT, n = 5 for caspase-9, cleaved caspase-3, and Bcl2,n = 3 for caspase-3. Data are presented as means ± SEM.
CPA, an inhibitor of SERCA2a, could induce the dysfunction of SERCA2a24. Our expriments confirmed that CPA could inhibit the activity of SERCA2a and the inhibition of SERCA2a did not affect the expression of ZFAS1 (Fig. S8). Conversely, ZFAS1 exhibited time-dependent inhibition effects on SERCA2a activities (Fig. S9). Furthermore, CPA could induce the resting calcium overload in the cardiomyocytes (Fig. S10a) and cause impairment of the protective effects of siZFAS1 on calcium homeostasis in hypoxia-treated cardiomyocytes (Fig. S10b).
ZFAS1-induced cytosolic Ca2+ overload causes cardiomyocyte apoptosis
It has been reported that BAPTA (ethane-N,N,N′,N′-tetraacetic acid) is a calcium chelator that resists cytosolic Ca2+ overload25. Our previous study had verified that ZFAS1 could induce cytosolic Ca2+ overload18. At present, forced expression of ZFAS1 with transfection of ZFAS1-P directly caused injured cell viability in non-hypoxic NMCMs. These effects of ZFAS1 were effectively reversed by pre-treatment with BAPTA (Fig. 5a, b). At the molecular level, BAPTA inhibited the ZFAS1 overexpression-mediated upregulation of pro-apoptotic proteins Bax, caspase-3, and capase-9 (Fig. 5c–e). Our previous study identified a functional domain of ZFAS1 named ZFAS1-FD, which showed similar effects on modulation of SERCA2a via direct binding. Similarly, ZFAS1-FD caused decreased cell viability, compromised mitochondrial membrane potential and increased apoptosis of cardiomyocytes, which could been ameliorated by BAPTA (Fig. 5f–k). Furthermore, compared with the ZFAS1-FD overexpressed NMCMs, the expression of apoptosis-associated proteins caspase-3, caspase-9, and Bax were significantly decreased in the BAPTA-treated NMCMs (Fig. 5l–n).
a MTT assays were performed to determine the cell viability of cardiomyocytes transfected with ZFAS1-P, with and without pretreatment with BAPTA. **P < 0.01 vs. control group, ##P < 0.01 vs. ZFAS1-P, n = 15. b LIVE/DEAD Viability/Cytotoxicity Kit stains were used to detect the viability of ZFAS1-overexpressing cardiomyocytes, with or without pretreatment with BAPTA. **P < 0.01 vs. control group, ##P < 0.01 vs. ZFAS1-P, n = 5. Magnification, ×200. c–e Western-blot analysis of the expression of apoptosis related proteins. **P < 0.01 vs. control group, ##P < 0.01 vs. ZFAS1-P, n = 4–5. f MTT assays were performed to determine the effects of BAPTA on the cell viability of ZFAS1-overexpressing cardiomyocytes. *P < 0.05 vs. control group, #P < 0.05 vs. ZFAS1-FD, n = 10. g, h LIVE/DEAD Viability/Cytotoxicity Kit Stains were used to determine the viability of cardiomyocytes transfected with ZFAS1-FD, with or without pretreatment with BAPTA. **P < 0.01 vs. control group, ##P < 0.01 vs. ZFAS1-FD, the results are expressed as the means ± SEM of three independent experiments. Magnification, ×200. i Effects of BAPTA on the mitochondrial membrane potential of ZFAS1-overexpressing cardiomyocytes were determined by JC-1 staining. Magnification, ×1200. Similar results were consistently observed in another two batches of cells. j, k ZFAS1-FD-induced cardiomyocyte apoptosis and BAPTA pretreatment could rescue its effects, which was validated by TUNEL assay. Blue, DAPI staining for nucleus; green, TUNEL-positive staining for apoptotic cells. *P < 0.05 vs. control group, #P < 0.05 vs. ZFAS1-FD, the results are expressed as the means ± SEM of three independent experiments. Magnification, ×200. l–n Western-blot analysis of the expression of apoptosis related proteins. ZFAS1-FD increased the expression of Bax, caspase-3, and caspase-9, and BAPTA pretreatment could suppress this change.*P < 0.05, **P < 0.01 vs. control group, #P < 0.05, ##P < 0.01 vs. ZFAS1-FD, n = 4 for caspase-3 and caspase-9, n = 5 for Bax. Data are presented as means ± SEM.
Discussion
This study presents the following significant results: (1) knockdown of ZFAS1 protects cardiomyocytes against MI and hypoxia treatment-induced apoptosis; (2) upregulation of ZFAS1 expression induces cardiomyocyte apoptosis; (3) ZFAS1 induces cardiomyocyte apoptosis by inhibiting SERCA2a and causing cytosolic Ca2+ overload.
To further explore the role of ZFAS1 on the heart, ZFAS1 cardiac-specific knock-in mice were constructed. Consistent with our previous study, ZFAS1 knock-in mice (TG) showed impaired cardiac function, deleteriously altered Ca2+ homeostasis, and repressed expression of SERCA2a. At the subcellular level, ZFAS1 induced mitochondrial swelling and markedly decreased the mitochondrial membrane potential. At the molecular level, ZFAS1 activated the mitochondria apoptosis pathway, which could be nearly abolished by a calcium chelator. The effects of ZFAS1 were readily reversible upon knockdown of this lncRNA. Notably, ZFAS1-FD mimicked the effects of the full-length ZFAS1 in regulation of cardiomyocyte apoptosis. Based upon these findings, we concluded that ZFAS1, an endogenous SERCA2a inhibitor, induces mitochondria-mediated apoptosis by causing cytosolic Ca2+ overload. Therefore, anti-ZFAS1 might be considered as a new therapeutic strategy for protecting cardiomyocytes from MI-induced apoptosis.
In the past few years, we have devoted ourselves to the study of lncRNA-ZFAS1 in cardiovascular diseases. By collecting and detecting the blood samples from AMI patients, non-AMI control subjects and healthy volunteers, we identified that ZFAS1 was a potential biomarker for MI17. Our recently published study suggested that lncRNA-ZFAS1 is a SERCA2a inhibitor that causes intracellular Ca2+ overload and contractile dysfunction in a mouse model of MI18. In this study, we further demonstrated the inhibitory effect of ZFAS1 on SERCA2a by using cardiac-specific ZFAS1 knock-in mice. During the course of this study, a research article on ZFAS1 in the heart was published, wherein the authors demonstrated that ZFAS1 promotes apoptosis by acting as a ceRNA to reduce the functional availability of miR-150 and increase the level of C-reactive protein26. As a key regulatory factor of MI, we believe that the regulation mechanism of ZFAS1 is necessarily diverse. The finding in the present study that ZFAS1 caused intracellular Ca2+ overload, thereby inducing cardiomyocyte apoptosis is another alternative mechanism for its pro-apoptotic function.
In fact, there was a limitation of our present study. In the cardiac tissue of TG, the mRNA level of SERCA2a was decreased. To verify the question how ZFAS1 altered the mRNA level of SERCA2a, we performed the bioinformatic prediction. By computer blasting and RNAfold website calculating, we found that ZFAS1 could bind to the mRNA of SERCA2a both at the CDS region (a total of 94 base sequences from 413 to 507 in ZFAS1 are complementary to 93 sequences from 1990 to 2083 in SERCA2a CDS region) and the 3′UTR (a total of 178 base sequences from 564 to 742 in ZFAS1 are complementary to 165 sequences from 24 to 189 in SERCA2a 3′UTR region) with lower free energy. The results above indicated that ZFAS1 might interact with mRNAs through direct antisense complementary to induce degradation of the SERCA2a mRNA. However, it is lack of rigorous studies to elucidate the exact mechanisms. We believed that mechanism of lncRNAs regulating mRNAs was worthy of further study.
The findings in the present study, the published studies by our laboratory and other research group indicated that lncRNA-ZFAS1 plays important roles in the setting of MI. By inhibiting SERCA2a, ZFAS1 not only caused cardiac dysfunction but also cardiomyocytes apoptosis during MI. Particularly, knockdown of ZFAS1 showed protective effects on the ischemia myocardium or hypoxia NMCMs This study strengthened the evidence that ZFAS1 might be a potential target for the treatment of MI.
References
Castro-Dominguez, Y., Dharmarajan, K. & McNamara, R. L. Predicting death after acute myocardial infarction. Trends Cardiovasc. Med. 28, 102–109 (2018).
Mehta, L. S. et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 133, 916–947. (2016).
Burgos, J. I., Morell, M., Mariangelo, J. I. E. & Vila Petroff, M. Hyperosmotic stress promotes endoplasmic reticulum stress-dependent apoptosis in adult rat cardiac myocytes. Apoptosis 24, 785–797 (2019).
Zhou, Y., Richards, A. M. & Wang, P. MicroRNA-221 is cardioprotective and anti-fibrotic in a rat model of myocardial infarction. Mol. Ther. Nucleic Acids 17, 185–197 (2019).
Elmore, S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516 (2007).
Charununtakorn, S. T., Shinlapawittayatorn, K., Chattipakorn, S. C. & Chattipakorn, N. Potential roles of humanin on apoptosis in the heart. Cardiovasc. Ther. 34, 107–114. (2016).
Portt, L., Norman, G., Clapp, C., Greenwood, M. & Greenwood, M. T. Anti-apoptosis and cell survival: a review. Biochim. Biophys. Acta 1813, 238–259. (2011).
Schwabe, R. F. & Luedde, T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 15, 738–752 (2018).
Liang, Q. et al. The role of IP3R-SOCCs in Cr(vi)-induced cytosolic Ca2+ overload and apoptosis in L-02 hepatocytes. Toxicol. Res. 7, 521–528 (2018).
Lim, W., An, Y., Yang, C., Bazer, F. W. & Song, G. Chrysophanol induces cell death and inhibits invasiveness via mitochondrial calcium overload in ovarian cancer cells. J. Cell. Biochem. 119, 10216–10227 (2018).
Jarroux, J., Morillon, A. & Pinskaya, M. History, discovery, and classification of lncRNAs. Adv. Exp. Med. Biol. 1008, 1–46 (2017).
Zhang Y, Du W, Yang B. Long non-coding RNAs as new regulators of cardiac electrophysiology and arrhythmias: Molecular mechanisms, therapeutic implications and challenges. Pharmacol. Ther. 2019;203:107389.
Wang, M., Jiang, S., Yu, F., Zhou, L. & Wang, K. Noncoding RNAs as molecular targets of resveratrol underlying its anticancer effects. J. Agric. Food Chem. 67, 4709–4719 (2019).
Yang, H., Li, G., Cheng, B. & Jiang, R. ZFAS1 functions as an oncogenic long non-coding RNA in bladder cancer. Biosci. Rep. 38, pii: BSR20180475 (2018).
Xie, S., Ge, Q., Wang, X., Sun, X. & Kang, Y. Long non-coding RNA ZFAS1 sponges miR-484 to promote cell proliferation and invasion in colorectal cancer. Cell Cycle 17, 154–161 (2018).
Pan, L. et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J. Cancer Res. Clin. Oncol. 143, 991–1004 (2017).
Zhang, Y. et al. Reciprocal changes of circulating long non-coding RNAs ZFAS1 and CDR1AS predict acute myocardial infarction. Sci. Rep. 6, 22384 (2016).
Zhang, Y. et al. LncRNA ZFAS1 as a SERCA2a inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ. Res. 122, 1354–1368 (2018).
Wan, N. et al. Toll-interacting protein contributes to mortality following myocardial infarction through promoting inflammation and apoptosis. Br. J. Pharmacol. 172, 3383–3396. (2015).
Takahara, S. et al. New Noonan syndrome model mice with RIT1 mutation exhibit cardiac hypertrophy and susceptibility to β-adrenergic stimulation-induced cardiac fibrosis. EBioMedicine 42, 43–53 (2019).
Zhang, Y. et al. Long non-coding RNA CCRR controls cardiac conduction via regulating intercellular coupling. Nat. Commun. 9, 4176 (2018).
Hu, X. L. et al. MicroRNA-132 regulates total protein of Nav1.1 and Nav1.2 in the hippocampus and cortex of rat with chronic cerebral hypoperfusion. Behav. Brain Res. 366, 118–125 (2019).
Wang, L., De Jesus, N. M. & Ripplinger, C. M. Optical mapping of intra-sarcoplasmic reticulum Ca2+ and transmembrane potential in the Langendorff-perfused rabbit heart. J. Vis. Exp. 103, 53166 (2015).
Szentesi, P., Pignier, C., Egger, M., Kranias, E. G. & Niggli, E. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ. Res. 95, 807–813 (2004).
Collatz, M. B., Rüdel, R. & Brinkmeier, H. Intracellular calcium chelator BAPTA protects cells against toxic calcium overload but also alters physiological calcium responses. Cell Calcium 21, 453–459 (1997).
Wu, T. et al. Knockdown of long non-coding RNA-ZFAS1 protects cardiomyocytes against acute myocardial infarction via anti-apoptosis by regulating miR-150/CRP. J. Cell. Biochem. 118, 3281–3289 (2017).
Funding
This work was supported in part by the National Key R&D Program of China (2017YFC1702003), the National Natural Science Foundation of China (81670238/81773735/81570357).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by S. Lavandero
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiao, L., Li, M., Shao, Y. et al. lncRNA-ZFAS1 induces mitochondria-mediated apoptosis by causing cytosolic Ca2+ overload in myocardial infarction mice model. Cell Death Dis 10, 942 (2019). https://doi.org/10.1038/s41419-019-2136-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-2136-6
This article is cited by
-
Mettl13 protects against cardiac contractile dysfunction by negatively regulating C-Cbl-mediated ubiquitination of SERCA2a in ischemic heart failure
Science China Life Sciences (2023)
-
Long Non-coding RNA Involved in the Pathophysiology of Atrial Fibrillation
Cardiovascular Drugs and Therapy (2023)
-
Connexin 43 hyper-phosphorylation at serine 282 triggers apoptosis in rat cardiomyocytes via activation of mitochondrial apoptotic pathway
Acta Pharmacologica Sinica (2022)
-
Lnc Tmem235 promotes repair of early steroid-induced osteonecrosis of the femoral head by inhibiting hypoxia-induced apoptosis of BMSCs
Experimental & Molecular Medicine (2022)
-
Long Noncoding RNAs Involved in Cardiomyocyte Apoptosis Triggered by Different Stressors
Journal of Cardiovascular Translational Research (2022)