Abstracts
Wnt/ Wingless (Wg) is essential for embryonic development and adult homeostasis in all metazoans, but the mechanisms by which secreted Wnt/Wg is processed remain largely unknown. A Drosophila Sol narae (Sona) is a member of A Disintegrin And Metalloprotease with ThromboSpondin motif (ADAMTS) family, and positively regulates Wg signaling by promoting Wg secretion. Here we report that Sona and Wg are secreted by both conventional Golgi and exosomal transports, and Sona cleaves extracellular Wg at the two specific sites, leading to the generation of N-terminal domain (NTD) and C-terminal domain (CTD) fragments. The cleaved forms of extracellular Wg were detected in the extracellular region of fly wing discs, and its level was substantially reduced in sona mutants. Transient overexpression of Wg-CTD increased wing size while prolonged overexpression caused lethality and developmental defects. In contrast, Wg-NTD did not induce any phenotype. Moreover, the wing defects and lethality induced by sona RNAi were considerably rescued by Wg-CTD, indicating that a main function of extracellular Sona is the generation of Wg-CTD. Wg-CTD stabilized cytoplasmic Armadillo (Arm) and had genetic interactions with components of canonical Wg signaling. Wg-CTD also induced Wg downstream targets such as Distal-less (Dll) and Vestigial (Vg). Most importantly, Cyclin D (Cyc D) was induced by Wg-CTD but not by full-length Wg. Because Sona also induces Cyc D in a cell non-autonomous manner, Wg-CTD generated by Sona in the extracellular region activates a subset of Wg signaling whose major function is the regulation of cell proliferation.
Similar content being viewed by others
Introduction
Cellular communication via components in the extracellular matrix (ECM) is essential for cell survival and proliferation as well as differentiation. Extracellular proteases play important roles in regulating activity, localization and stability of the ECM proteins1,2,3. Despite the importance of these proteases, their specific functions are still largely unexplored. ADAMTS family contains extracellular proteases that are present only in metazoans4,5. Six and nineteen members have so far been identified in flies and mammals, respectively6,7. Mammalian ADAMTSs are involved in cell proliferation, angiogenesis and organogenesis, so their malfunctions result in various diseases such as cancer, arthritis, and arteriosclerosis7,8,9. An ADAMTS Sol narae (Sona) is essential for fly development10. Loss of sona decreases the level of extracellular Wg, and sona exhibits positive genetic interaction with wntless (wls) that encodes a cargo protein for Wg10,11,12. Therefore, intracellular Sona seems to cooperate with Wls in Wg secretion10. We recently reported a new function of extracellular Sona in cell survival and cell proliferation13. sona has genetic interactions with cell death-related genes such as Death-associated inhibitor of apoptosis (Diap1) and reaper. Interestingly, Sona upregulates Cyclin D (Cyc D) in a cell non-autonomous manner, and increases tissue size. Cyc D is a G1 Cyclin to initiate the cell cycle by responding to the mitogen signals14. Therefore, it is possible that extracellular Sona generates a yet unidentified signaling molecule that induces Cyc D in the signal-receiving cells.
Wnt family is essential for animal development, and has been extensively studied since a mutant of fly Wg, the homolog of vertebrate Wnt1, was described a century ago15,16,17. Wnt is secreted by both conventional Golgi-mediated transport and exosomal secretion pathway18,19,20. Interaction between Wnt and Frizzled (Fz) receptors initiates a cascade of intracellular responses in the responding cells that lead to downstream gene expression21,22. In flies, Wg is involved in cell proliferation, differentiation, and survival by inducing Wg effector components including Vestigial (Vg), Distal-less (Dll) and Senseless (Sens)23,24,25,26. In mammals, Wnt signaling promotes cell proliferation by transcriptional activation of multiple target genes such as c-Myc and Cyc D27,28,29 and its malfunction leads to various diseases such as cancer, neurodegenerative diseases, inflammatory disease, and diabetes30,31,32.
We asked the role of extracellular Sona in this study and found that Sona generates NTD and CTD fragments of Wg by cleaving extracellular Wg. The Wg-CTD fragment was similar to full-length Wg in activating canonical Wg signaling but was dissimilar to full-length Wg in Cyc D induction, lack of Sens induction, and protein instability. Thus, one of the main functions of Sona is to generate Wg-CTD that carries out subsets of Wg signaling.
Results
Sona and Wg are secreted by both conventional Golgi and exosomal transports
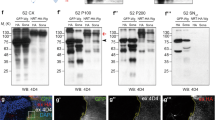
Sona is an ADAMTS protease secreted as an active form to the extracellular region10, and has biochemical and genetic interactions with Wg that is secreted by both conventional Golgi and exosomal transports18,19,20. As a first step toward understanding the role of extracellular Sona, we examined by which pathway Sona is secreted. To this end, we obtained conditioned media from the culture of S2 sona-HA cell line, and precleared it to obtain the initial supernatant fraction, SN0. Centrifugation of SN0 at 100,000×g yielded two fractions: a supernatant fraction (SNΔ) that contains soluble proteins secreted by Golgi transport, and a pellet fraction (P100) that contains exosomes19,33. The cell extract (CX) contained both full-length Sona-HA (red arrow) and the active form of Sona-HA lacking its pro-domain (black arrow), while the SNΔ and P100 fractions contained only the active form of Sona-HA (Fig. 1a). Our data demonstrate that Sona is also secreted by both Golgi and exosomal transports (Fig. 1b). To prove further that Sona is secreted by exosomal pathway, we examined the P100 fraction of Sona-HA in detail. Purity of the P100 fraction was verified by the presence of the exosomal markers Syntaxin 1A (Syx1A) and Alix as well as the absence of the ER marker Calnexin33,34 (Fig. 1c). Particulate structures with 70–250 nm diameter were detected in the P100 fraction of S2 sona-HA or S2 GFP-wg by Nanoparticle tracking analysis (NTA), and Sona and Wg were present in the fraction with 1.09–1.11 g / mL density in sucrose step gradient33 (Fig. 1d and Supplemental Fig. S1A-B). Furthermore, Sona-HA was also present on the outer surface of vesicles (Fig. 1e and Supplemental Fig. S1C, D). To confirm the presence of Sona on exosomes in vivo, we examined whether Sona-HA and the exosomal marker CD63-GFP colocalize in wing discs. Unlike the lysosomal markers (Supplemental Fig. S2), half of CD63-GFP-positive (+) vesicles contained Sona-HA detected by both anti-HA and Sona-Pro antibodies (52.3 ± 10.3%, n = 65, Supplemental Fig. S1E)10, and about half of these CD63+ Sona+ vesicles contained endogenous Wg (21.5 ± 2.1%, n = 65, Supplemental Fig. S1F). These results demonstrate that Sona is present on exosomes.
In all western analyses, blotting antibodies are indicated at the bottom of each panel. Fractions and the source of cells transfected with different constructs are written at the top of panels. S2 cells are derived from a macrophage-like lineage of fly embryonic cells78, and express neither Sona nor Wg10,19,33. Therefore, UAST-GFP-wg and UAST-sona-HA cDNA constructs were expressed by actin-Gal4 driver in S2 cells. HA is the preparation from cells transfected with the control UAST-HA vector. The pound signs (#) indicate the cleaved Wg fragments in the absence of Sona. These fragments may be generated by degradation during sample preparation or by some Wg-specific proteases endogenously expressed in S2 cells. a Full-length and processed forms of Sona-HA in cell extract (CX), SNΔ, and P100 fractions. The P100 fraction was four times more concentrated than the SNΔ fraction, and the equal volumes of concentrated samples were loaded for the blot. Therefore, the actual amount of Sona in the SNΔ fraction should be four times more than the one in the blot. The SNΔ fraction always contained more Sona than the P100 fraction. Full-length Sona and active Sona are marked with red arrows and black arrow, respectively. b Full-length GFP-Wg (GFP-WgFL) in CX, SNΔ, and P100 fractions. SNΔ and P100 fractions were concentrated equally and the same volumes were loaded. GFP-Wg was more abundant in the SNΔ fraction than the P100 fraction (red arrow)19,33. c Verification of CX, P100, SN0, and SNΔ fractions using the ER marker Calnexin and the exosomal markers Alix and Syntaxin 1A (Syx1A). d Diameter and number of vesicles in P100 fractions from S2 sona-HA cells measured by NTA. e A immuno-EM image of Sona-HA in the P100 fraction with anti-HA antibody. The diameter of vesicles with Sona-HA is about 120 nm. f–f” GFP-WgFL (83 kDa) and a GFP-Wg fragment (65 kDa) are indicated with red and black arrows, respectively. g, h The black arrow and the arrowhead indicate 65 and 60 kDa GFP-Wg fragments, respectively. The same blot was used for both (g) and (h). i A blot in (i) was exposed longer in (i’). The 41 kDa and the 23 kDa fragments are marked by the black arrows and the red arrowhead, respectively. Non-tagged WgFL are marked by red arrows. j The 23 kDa Wg-3XHA fragment are marked with the red arrowhead. This fragment was readily detected because it has three times HA epitope than 4D4 epitope. k Domain structures of full-length and cleaved forms of Wg (modified from a report79). Two cleavage sites (L1, L2) are indicated with scissors. The 4D4 epitopes are marked with red
The active form of extracellular Sona is essential for cleavage of the Wg linker region
Coimmunoprecipitation of Sona and Wg10 suggested that Sona may cleave Wg. To test this, we examined whether any small Wg fragments are generated in the presence of Sona. In fact, a 65 kDa fragment smaller than 83 kDa full-length GFP-Wg (GFP-WgFL) was detected by the 4D4 Wg antibody in both SNΔ and P100 fractions only when Sona was coexpressed with GFP-Wg (black arrows in Fig. 1f’, f”). Such Sona-dependent changes were not detected in CX (Fig. 1f and Supplemental Fig. S3A, B). Interestingly, anti-GFP antibody detected both 65 kDa and an additional 60 kDa fragment (black arrowhead in Fig. 1h), indicating that these two fragments have the N-terminal region where GFP is inserted (Fig. 1k). Since these 65 and 60 kDa fragments were not produced by the protease-dead SonaE475A or in the presence of a zinc chelator, EDTA, metalloprotease activity of Sona is essential for Wg cleavage (Fig. 1g, h and Supplemental Fig. S3A-C). We located the two cleavage sites designated as L1 and L2 in the Wg linker based on two features. First, the 65 kDa but not the 60 kDa fragment has the 4D4 epitope (Fig. 1g, h). Second, the 4D4 epitope is located between amino acids 229 and 360 of Wg35,36. Cleavage at L1 and L2 should produce fragments named NTDL1, CTDL1, NTDL2, and CTDL2 (Fig. 1k). NTDL1 and NTDL2 are 60 kDa and 65 kDa fragments, respectively. Meanwhile, a 23 kDa fragment was detected with the 4D4 antibody when untagged Wg or GFP-Wg was coexpressed with Sona (red arrowheads in Fig. 1i’ and Supplemental Fig. S3A’), or detected with the HA antibody when Sona and Wg-3XHA with three HA tags at the C-terminus37 were co-expressed (red arrowhead in Fig. 1j). Therefore, this 23 kDa fragment is CTDL1 because it contains both the C-terminal part of Wg and the 4D4 epitope (Fig. 1k). However, CTDL1 fragments generated from the untagged Wg or GFP-Wg were detectable only after long exposure, and the intensity of CTDL1 fragment was much lower than that of NTDL2 (Fig. 1i-i’ and Supplemental Fig. S2A’). Moreover, the CTDL2 fragment was never detected when Wg-3XHA was coexpressed with Sona (Fig. 1j). The low levels of CTDL1 and the absence of the CTDL2 may be due to protein instability, which will be addressed in Fig. 2.
A report on Wnt7a-NTD and Wnt7a-CTD constructs42 was used to select the site (red vertical line) for separating NTD and CTD. Domain diagrams of GFP-NTD in (a), Linker-GFP in (d), and Linker-CTD in (i), and putative cleaved products with calculated molecular weights and their corresponding protein bands in western blots are marked by arrows and arrowheads. No Sona-dependent cleavage occurred in CX (b, e, g, j). All uncleaved forms are marked with red arrows. a–c GFP-NTD generated from GFP-WgFL in (a) and western blots of GFP-NTD with or without Sona in (b–c). d–h Linker-GFP with and without Sona. Fragments of Linker-GFP cleaved at L1 and L2 sites are marked with black arrows and arrowheads in (e–h). i–k Linker-CTD with or without Sona. The black arrowheads indicate 12 kDa fragments generated by L2 cleavage. l–n In vitro assay for detection of cleaved Wg fragments from GST-linker-CTD expressed in E. coil. Black arrow indicates immunoprecipitated Sona-HA using anti-HA antibody and asterisks (*) indicate Rat IgG bands in (n). Red arrowhead indicates cleaved 18 kDa CTDL1 fragment in (m) and the pound signs (#) indicate the degraded product of GST-linker-CTD independent of active Sona. o, p The amount of cleaved Wg products increase proportionally to the amount of active Sona. Amounts of Wg and Sona are written on the top of panels. Precleared conditioned media prepared by centrifuging at 10,000×g were mixed and then the P100 fractions obtained from the mixture were analyzed in (o). 23 kDa fragments are marked by a red arrowhead in (o). P100 fractions from Wg-expressing S2 cells and those from Sona-expressing S2 cells were mixed, and then the P100 fractions obtained from those mixtures were analyzed in (p). 65 and 60 kDa GFP-Wg fragments are indicated by a black arrow and a black arrowhead, respectively in (p)
The linker of Wg is necessary and sufficient for Wg cleavage by Sona
To examine whether the linker is the only region required for Wg cleavage, we generated two constructs, GFP-NTD and linker-GFP that encode 53 kDa GFP-NTD without the linker region and 48 kDa Linker-GFP with the linker region fused to GFP, respectively (Fig. 2a, d). When GFP-NTD and Sona-HA were coexpressed, Sona-dependent cleavage was not detected (Fig. 2c). In contrast, we found all cleavage products such as a 42 kDa L1 cleavage product (black arrows), a 34 kDa L2 cleavage product (red arrowheads), and a 12 kDa L2 cleavage product (black arrowheads) when Linker-GFP and Sona-HA were coexpressed (Fig. 2f, h). Furthermore, Wg cleavage by Sona occurred when Sona and Wg were prepared from different cells or different secretion pathways (Supplemental Fig. S3D-F). Therefore, the linker region is necessary and sufficient for Wg cleavage by Sona regardless of origin.
The CTD domain of Wg-CTD fragments is responsible for protein instability
We previously mentioned that both CTDL1 and CTDL2 fragments might be unstable (Fig. 1i). However, the 42 kDa L1 Linker-GFP fragment that is equivalent to the 23 kDa Wg-CTDL1 fragment was readily detected (Compare Fig. 1i to Fig. 2h). This raised a possibility that the CTD domain itself is responsible for instability of Wg-CTD fragments. To test this, we generated linker-CTD that encodes 29 kDa Linker-CTD and compared its Sona-dependent cleavage products to those of Linker-GFP (Fig. 2i). When Linker-CTD and Sona were coexpressed, a 12 kDa 4D4-positive L2 cleavage product was detected (black arrowheads) but the expected 23 kDa CTDL1 was not detected even with multiple attempts (Fig. 2k). Moreover, the 34 kDa L2 linker-GFP fragment was detected (red arrowhead in Fig. 2f), which is equivalent to the 17 kDa Wg-CTDL2 fragment. As shown before, this 17 kDa Wg-CTDL2 fragment was never detected throughout this study (Fig. 1j, k). Therefore, both Wg-CTD fragments are less stable than their equivalents due to the CTD itself.
Wg is directly cleaved by Sona
To test the enzyme-substrate relationship between Sona and Wg, we asked whether Sona cleaves Wg in vitro. Since the NTD of Wg is too hydrophobic to be expressed in E. coli, we generated GST-linker-CTD (Fig. 2l). We hypothesized that Sona may cleave GST-linker-CTD because the linker is sufficient for cleavage by Sona (Fig. 2l). When GST-linker-CTD purified from E. coli was incubated with active Sona purified from the SNΔ fraction of S2 sona-HA (Supplemental Fig. S4A), the 4D4 antibody detected a Sona-dependent 18 kDa fragment (red arrowhead in Fig. 2m and Supplemental Fig. S4B; black arrow in Fig. 2n and Supplemental Fig. S4C). This 18 kDa fragment is the L1 cleavage product that is equivalent to the 23 kDa Wg-CTDL1 fragment, but is smaller because the CTD domain in the GST-linker-CTD is not glycosylated (Supplemental Fig. S4D). This suggests that Wg is a substrate of Sona.
If Sona directly cleaves Wg, the amount of cleaved Wg would positively correlate with that of Sona. To test this, the fixed amount of SN0 containing Wg was incubated with the increasing amounts of SN0 containing Sona-HA. As expected, the amount of Wg-CTDL1 fragment positively correlated with that of Sona (Fig. 2o). When the fixed amount of the P100 containg GFP-Wg was incubated with the increasing amounts of the P100 containing Sona-HA, the amount of Wg-NTDL1 and Wg-NTDL2 fragments also proportionally increased (Fig. 2p). This result is consistent with direct cleavage of Wg by Sona.
Sona is required for cleavage of extracellular Wg in vivo
We next asked whether cleavage of extracelluar Wg also occurs in vivo. Wg is highly expressed along the DV midline of wing discs38,39,40. To detect cleaved Wg forms, we examined extracellular Wg-HA pattern in the DV midline of wg[KO; Wg-HA]41. Assuming Sona cleaves Wg at L1 and L2 cleavage sites in vivo, we expected to detect extracellular structures including Wg-HAFL and four additional Wg fragments except NTDL1 in wg[KO; Wg-HA] wing discs using anti-HA and 4D4 antibodies (Fig. 3a). We found not only yellow structures (HA+ 4D4+) that contain full-length Wg but also the green (HA+ 4D4–) and the red structures (HA– 4D4+) that represent cleaved Wg fragments (Fig. 3a, e). These cleaved structures were also detected in wg > GFP-wg discs (Supplemental Fig. S5). Thus, cleaved Wg fragments were present in the extracellular region.
a Domain structures of Wg-HA and cleaved products drawn with red, green, and yellow bars. The Grey bar indicates undetectable NTDL1 fragment. The 4D4 epitope is present in the linker region. The HA (green) and 4D4 (red) signals match with the colors in the confocal images. b, c Extracellular staining of a homozygous wg[KO;Wg-HA] wing disc at the basal ECM. In the wg[KO; Wg-HA] strain, the endogenous wg gene is replaced by a wg-HA transgene that fully supports fly development41. Extracellular Wg-HA was detected by anti-4D4 and anti-HA antibodies. Extracellular HA signals were detected only in wg[KO; Wg-HA] (c) but not in CS wing discs (b). These two samples were stained at the same time under the same condition. d–g Extracellular 4D4 and HA staining of wg[KO; Wg-HA] and sona47/sona18 wg[KO; Wg-HA] wing discs. The sona18 and sona47 encode Sona with an internal deletion and a C-terminal truncation, respectively, and both cause pupal lethality10. Representative green (HA+ 4D4– including CTDL2), red (HA– 4D4+ including NTDL2 or the linker fragment), and yellow (HA+ 4D4+ including WgFL or CTDL1) signals are marked by an arrowhead, an arrow, and a circle, respectively. The squared regions in (d) and (f) are magnified in (e) and (g), respectively. h The proportions of blue bars representing HA+4D4+ structures and red bars representing both HA+4D4– and HA– 4D4+ structures. 4 wg[KO;Wg-HA] discs and 4 sona47/sona18 wg[KO; Wg-HA] discs were used for counting extracellular structures. The total numbers of counted structures are written on the top of the graph. The average percentages of counted vesicles are shown on the right. Graphs are displayed as mean ± S.E.M, where ∗∗p < 0.01. Scale bars for (b) and (c), 60 μm; (d) and (f), 40 μm; (e) and (g), 5 μm
If Sona plays a major role in the cleavage of WgFL, sona mutants should have more WgFL than wild-type. To test this, we compared the percentage of yellow structures (HA+ 4D4+) in sona18/ sona47 wg[KO; Wg-HA] discs with that in control wg[KO; Wg-HA] discs (Fig. 3d–h). While 74.1% (648/875) were yellow in sona18/sona47 discs, only 32.0% (460/1436) were yellow in wild-type discs (Fig. 3h). This suggests that Wg cleavage occurred at a lesser extent in the sona discs. This supports that Sona is a major player in cleavage of Wg in vivo.
Prolonged overexpression of Wg-CTD induces morphological defects and lethality
We reasoned that at least one of the cleaved forms of Wg should be active because Sona positively regulates Wg signaling10. To test which Wg fragment is active, we performed luciferase assay in S2R+ cells that expressed Wg-NTD or Wg-CTD. Neither Wg-NTD nor Wg-CTD, however, showed any Wg activity (Supplemental Fig. S6A). This was unexpected because artificially engineered Wnt7a-CTD is reported to be active in TopFlash reporter assay42. It is possible that S2 R+ cells lack some essential components that are required for Wg-CTD activity43, or Wg-CTD is too unstable in S2 R+ cell culture (Supplemental Fig. S6). Instead of finding the better condition for luciferase assay, we decided to test the activity of Wg fragments in vivo with UAS-GFP-wg-NTD and UAS-wg-mycCTD transgenic flies (Fig. 2a and Supplemental Fig. S7A).
Overexpression of GFP-Wg-NTD with engrailed (en)-Gal4 or nubbin (nub)-Gal4 produced no phenotypes (Fig. 4b and Supplemental Fig. S7B). In contrast, en > wg-mycCTD and nub > wg-mycCTD wings were small and deformed (Fig. 4a, d, k, l), and the posterior region of en > wg-mycCTD wing discs were smaller than that of control discs (Supplemental Fig. S7K, L). Furthermore, cubitus interruptus (ci) > wg-mycCTD eyes were small and rough (Supplemental Fig. S7N, O). Expression of Wg-mycCTD by actin-Gal4 or tubulin-Gal4 induced early larval lethality (n > 40) but that of GFP-wg-NTD produced no lethality. Expression of the untagged Wg-NTD and Wg-CTD also generated phenotypes similar to the tagged counterparts (Fig. 4c, e and Supplemental Fig. S7A, E, F, J, M). Taken together, Wg-CTD but not NTD is an active Wg form in vivo.
The genotypes of female wings are indicated at the lower left of each panel. a The en-Gal4 wing at 18 °C as a control. b, c Both en > GFP-wg-NTD wing in (b) and en > wg-NTD (no tag) wing in (c) were normal. d, e prolonged expressions of Wg-CTD in en > wg-mycCTD flies (d) and en > wg-CTD (no tag) flies (e) induce deformed wings with smaller posterior region than the control wing in (a) similar to phenotypes by prolonged expression of Wg48,49. f en > wg-mycCTD, Gal80ts wings from flies cultured for 10 h at 30 °C during the mid-third instar stage for transient Wg-mycCTD expression. Outline of the control wing in (a) is drawn in (f). g The average wing size of en > wg-mycCTD, Gal80ts and en > wg-CTD, Gal80ts flies (n = 31 each) cultured for 10 h at 30 °C are shown in bar graphs as mean ± S.E.M. h, i PH3 pattern in mid-3rd instar wing discs of ptc > GFP and ptc > GFP, wg-mycCTD. An arrow indicates increase in PH3 staining along the ptc region in (i). j The graph represents the average number of PH3 positive signals in the ptc region of ptc > GFP and ptc > GFP, wg-mycCTD wing discs (n = 10). k–r Wg-mycCTD expression rescues sona RNAi phenotypes. All nub > sona RNAi wings are small and malformed in (m). Wing phenotypes of nub > sona RNAi were rescued by coexpression of Wg-mycCTD in (n). wg > wg-myc-CTD wings in (p) did not show any phenotype similar to the control wg-Gal4 wings in (o). wg > sona RNAi (n = 71) had notched wing phenotype in (q), but wg > sona RNAi, wg-mycCTD had normal wings (n = 68) in (r). s Wg-mycCTD expression partially suppressed the lethality induced by sona RNAi. Adult flies, pupal lethal and embryonic to larval lethal in percentages are drawn in colored bars. 23% of nub > sona RNAi flies were embryonic to pupal lethal, but only 12% of nub > sona RNAi, wg-mycCTD flies were embryonic to pupal lethal (n = 160 each). Similarly, none of ptc > sona RNAi flies but 12% of ptc > sona RNAi, wg-mycCTD flies (n = 140) survived to adulthood and their lethal stage was also significantly delayed from embryonic to pupal stage (Supplemental Fig. S7A-D). Scale bar, 100 μm, ∗∗p < 0.01; ∗∗∗p < 0.001
Transient overexpression of Wg-CTD stimulates cell proliferation
Wg stimulates cell proliferation as a mitogen44,45, and moderate Wg overexpression increases the number of phosphohistone 3 (PH3)-positive cells44,46. To check whether Wg-CTD also induces cell proliferation, we transiently expressed Wg-CTD using Gal80ts in order to avoid cell death or cell cycle arrest by prolonged Wg signaling47,48,49. Indeed, expression of Wg-mycCTD or untagged Wg-CTD for ten hours increased wing size by 17.6 and 31.3%, respectively (n = 31, Fig. 4f, g). Likewise, the number of PH3-positive cells was increased along the ptc region compared to control ptc > GFP discs (Fig. 4h–j). Therefore, Wg-CTD is able to promote cell proliferation.
Wg-CTD rescues the loss of sona phenotypes
Wg-CTD would rescue the loss-of-function phenotypes of extracellular Sona if generation of Wg-CTD is a main function of extracellular Sona. To test this, sona RNAi-111-4 (sona RNAi, hereafter) was coexpressed with Wg-mycCTD and the wing phenotype and lethality were compared to those of sole sona RNAi expression10. All nub > sona RNAi wings were small, wrinkled or both10 (n = 95, Fig. 4k, m) but 68% of nub > sona RNAi, wg-mycCTD wings were normal (n = 109, Fig. 4n, s). Notched wing phenotype was observed in 55% of wg > sona RNAi (n = 71) but only in 6% of wg > wg-mycCTD, sona RNAi (n = 68) flies (Fig. 4o–r). Furthermore, notched wing phenotype of ptc > wg-mycCTD was rescued in ptc > sona RNAi, wg-mycCTD flies (Supplemental Fig. S8E-G). Therefore, Wg-CTD expression rescued loss of sona phenotypes.
Wg-CTD activates canonical Wg signaling
Cytoplasmic Arm becomes stabilized by activation of canonical Wg signaling50. Because Wg-CTD expression rescued the lethality and wing defects induced by arm RNAi, Sgg or dTCFDN expression (Fig. 5a–p), we tested whether Wg-CTD stabilizes the cytoplasmic Arm. In fact, ci > GFP, wg-mycCTD wing discs had the increased level of Arm in the anterior region (Fig. 5q, r). More Arm was also present in the CX of nub > wg-mycCTD compared to control nub-Gal4 wing discs (Fig. 5s).
a–p Adult female wings are shown with their genotypes indicated at the lower left of each panel. All flies were cultured at 18 °C except flies in (i), (j) and (k) cultured at 25 °C. The wg-Gal4 in (a) and nub-Gal4 in (e) wings as controls. The wg > wg-mycCTD wings were normal in (b), and the nub > wg-mycCTD wings were smaller in (f). The wg > arm RNAi flies in (c) and nub > arm RNAi flies in (g) were mostly pupal lethal but a few escapers (<1%) had severely malformed and small wings (n > 150). Co-expression of Wg-mycCTD rescued the wing size and morphology in (d) wg > arm RNAi, wg-mycCTD flies (2 out of 48) and (h) nub > arm RNAi, wg-mycCTD flies (8 out of 58). The nub > Sgg wings in (j) were small, and were rescued to normal wings by Wg-CTD co-expression (19 out of 81) in (k). The wg > dTCFDN flies in (m) (n > 100) were 100% pupal lethal with severely malformed wings but the wg > dTCFDN, wg-mycCTD wings were larger (3 out of 26) in (n). The c96 > dTCFDN flies in (o) had notched wings but c96 > dTCFDN, wg-mycCTD flies were normal (37 out of 50) in (p). q, r Arm in the wing discs of ci > GFP in (q) and ci > GFP, wg-mycCTD in (r) larvae. An arrow in (r’) indicates the anterior region where Arm is increased. s Western analysis to detect Arm in the CX prepared from nub > wg-mycCTD wing discs. α-Tubulin was used as a loading control. A red arrow indicates full-length Wg-mycCTD, and a black arrow indicates a cleaved fragment that may be equivalent to the L1 cleavage product of Wg-mycCTD. As previously shown, Wg-CTD L2 fragment was not detected. Scale bar, 100 μm
We next examined whether Wg-CTD increases levels of Wg effector proteins, Vg and Dll that are induced by canonical Wg signaling51,52,53,54. Transient expression of Wg-CTD by en-Gal4 increased the level of Vg and Dll in the posterior region of wing discs (Fig. 6a–d), and Wg-CTD-expressing flp-out clones had higher level of Dll (Fig. 6e–g). The untagged Wg-CTD expression in en > wg-CTD discs also increased the level of Dll (Supplemental Fig. S9B). GFP-Wg-NTD and untagged Wg-NTD failed to change the level of Wg effector proteins (Fig. 6h, i and Supplemental Fig. S9A). Therefore, Wg-CTD is a new form of active Wg that induces canonical Wg signaling.
a–d The flies were shifted from 18 °C to 30 °C for 10 h during the late second and early third larval instar for transient expression. The en > GFP, Gal80ts discs did not show any change in Vg and Dll in (a) and (c), but the en > GFP, Gal80ts, wg-mycCTD discs showed increased Vg and Dll in the posterior region marked by arrows in (b) and (d). e–g Analysis of GFP+ clones that overexpress Wg-CTD in hsflp; actin > y > Gal4; UAS-GFP (e) and hsflp; actin > y > Gal4; UAS-GFP/UAS-wgmycCTD (f) flies. GFP+ control clones in (e) showed no change but GFP+ Wg-CTD+ clones in (f) had higher level of Dll. The boxed region in (f) is magnified in (g). h, i en > GFP-wg-NTD wing discs showed no change in the levels of Vg in (h) or Dll in (i). Scale bar, 100 μm except (g), 40 μm
Wg-CTD upregulates the level of Cyc D for cell proliferation
Overexpressed WgFL partially rescued the lethal phenotype of wg mutants but Wg-CTD did not (Supplemental Fig. 9C-E). This demonstrates that Wg-CTD can carry out only subsets of Wg signaling and so called ‘Wg signaling’ is induced by combined activity of both WgFL and cleaved Wg-CTD. An important question is then whether Wg-CTD has any unique functions unshared by WgFL. We hypothesized that Wg-CTD may be more specialized for cell proliferation than WgFL because Sona increases the level of Cyc D13. Indeed, Cyc D was upregulated by prolonged expression of Wg-CTD in the anterior region of ci > wg-mycCTD wing discs and by transient expression of Wg-CTD in the posterior region of en > wg-mycCTD, Gal80ts discs cultured for 12 h at 30 °C (Fig. 7a, b; Supplemental Fig. S9F, G). GFP-WgFL or GFP-Wg-NTD expression, however, did not change the level of Cyc D (Fig. 7c, d). Taken together, Wg-CTD is able to induce Cyc D. Wg signaling plays an important role in neuronal differentiation by inducing sens in the DV margin of wing discs55,56,57. Transient expression of GFP-Wg increased the level of Sens in ptc > GFP-wg, Gal80ts discs, and induced ectopic sensory bristles in nub > GFP-wg, Gal80ts wings, which are consistent with previous reports58,59 (Fig. 7e–h). In contrast, transient expression of Wg-CTD did not induce ectopic Sens in ptc > wg-mycCTD Gal80ts and en > wg-mycCTD Gal80ts wing discs (Fig. 7i and Supplemental Fig. S9I). Wings of nub > wg-mycCTD, Gal80ts, nub > wg-mycCTD, and nub > wg-NTD flies also had no ectopic bristles (Fig. 7j and Supplemental Fig. S9K-L). Thus, Wg-CTD is not able to induce Sens unlike WgFL.
Transgenes were transiently expressed by shifting the culture temperature to 30 °C for 12 h during the late second and early third larval instar in all cases. a–d The level of Cyc D in wing discs. Arrows mark the region with higher level of Cyc D in en > GFP, wg-mycCTD discs in (b). No changes in control ci > GFP in (a), en > GFP-wg-NTD in (c) and en > GFP-Wg, Gal80ts in (d) discs. e–j Sens expression in wing discs and sensory bristle formation in adult wings. Sens level in control (e) and ptc > wg-mycCTD, Gal80ts wing discs in (i). The arrow indicates ectopic Sens by GFP-WgFL in ptc > GFP-wg, Gal80ts wing discs in (g). Expression of full-length GFP-Wg in (h) induced ectopic bristles in wing blades, but control in (f) and Wg-CTD expression in (j) did not induce any ectopic bristles. Scale bar, 100 μm
Discussion
We report here that Sona cleaves extracellular Wg into Wg-NTD and Wg-CTD, and the Wg-CTD is a new form of active Wg (Supplemental Fig. S10). Because Wg-CTD substantially rescued the sona loss-of-function phenotypes such as lethality and wing defects (Fig. 4), generation of Wg-CTD seems to be one of Sona’s major functions. Wnt modifications such as lipidation and glycosylation have been extensively studied, but Wnt cleavage has not been addressed except for the Xenopus Tiki protease. Tiki reduces Wnt secretion by cleaving the amino-terminal region of intracellular Wnt that is required for the lipidation of Wnt60. While Tiki aims to decrease the amount of secreted Wnt, Sona aims to generate a new active form of Wg from an already active WgFL.
Genetic interaction between wg-CTD and other Wg signaling components indicates that Wg-CTD activates Wg signaling similar to WgFL (Fig. 5). However, there are several differences between these two forms of Wg. First, Wg-CTD but not WgFL increased the level of Cyc D (Fig. 7). Overexpressed Cyc D-Cdk4 in flies accelerates cell division of undifferentiated cells such as wing disc cells61. Sona also induces Cyc D and promotes cell proliferation in a cell non-autonomous manner13. Therefore, Wg-CTD generated by extracellular Sona seems to induce Cyc D in the neighboring cells for cell proliferation. Second, both Wg-CTDL1 and Wg-CTDL2 are less stable than WgFL. Instability of Wg-CTD may be an essential feature because mitogens and their downstream components are often removed by degradation to prevent excessive cell proliferation62 (Fig. 4). Presence of Wg-CTDL2-like structures in wing discs (Fig. 3), however, implies that these Wg-CTDL2-like structures may be stabilized in vivo by ECM components to achieve spatiotemporal regulation of the mitogenic activity63,64. Third, Wg-CTD is not able to induce Sens (Fig. 7 and Supplemental Fig. S8). Sens expression in the DV midline is required for differentiation of wing margin bristles55,56,57, unlike Vg that is essential for cell proliferation and cell survival23,65.
The difference between the two Wg forms in Sens induction may be due to their differential affinity to Fz receptors, based on the report that NTD and CTD of vertebrate Wnts able to interact Fz receptors independently from each other with different affinity66. It has been proposed that Wnt is generated during evolution via the fortuitous fusion of two ancestral proteins analogous to its NTD, homologous to a class of lipid-interacting proteins, and CTD, homologous to a group of cytokines involved in cell signaling42,66,67. This explains why NTD mutants are unable to be secreted68, while CTD mutants are secreted but inactive69. Given the evolutionary conservation of the components of Wnt signaling, ADAMTSs may also be involved in the generation of functional Wnt-CTD in mammals70,71. We expect that further study on the relationship between Wnts and ADAMTSs will expand our understanding on Wnt signaling and Wnt-related diseases.
Materials and methods
Drosophila strains, transgenic lines and generation of ectopic clones
sona mutants, sona RNAi lines, UAS-sona, and UAS-sona-HA are described elsewhere10. The UAS-wg-mycCTD, UAS-GFP-wg-NTD, UAS-wg-CTD and UAS-wg-NTD flies were generated for this study. UAS-CD63-GFP72, UAS-GFP-wg73, wg[KO; Wg-HA]41, UAS-GFP-lamp74, wgGal475 and ci-Gal476 were kindly provided by other labs that produced them. All other lines were obtained from the Bloomington stock center.
DNA constructs
The pAc-GFP-wg and pAc-wg-3XHA were constructed by recombining the pAc5.1 vector with GFP-wg or wg-3XHA obtained from MK33-GFP-wg (a gift from J.P. Vincent, unpublished) or UAS-wg-3XHA37. To generate the GFP-wg-NTD (GFP-NTD) and wg-mycCTD constructs, a myc tag was inserted in the DNA corresponding to the region between Arg367 and Tyr368 in GFP-Wg. DNA fragments representing GFP-NTD (1–245) and mycCTD (1–22, 245–468) were then amplified by PCR and inserted into pUAST vectors by recombination cloning methods.
Cell lines, cell culture, and exosome preparation
Drosophila S2 tub-wg, S2R+, and S2 cell lines were obtained from DGRC. S2 GFP-wg, wg-3XHA, and sona-HA stable cell lines were generated by selection under 2.5 μg/ml hygromycin B (Invitrogen) as follows. Drosophila S2 cell were grown in M3 media (Sigma-Aldrich) supplemented with 10% IMS (Sigma-Aldrich) at 25 °C. Stable cell lines were grown with hygromycin in 10% IMS M3 media, and S2 tub-wg cells were cultured in 10% FBS M3 media. Transfections were carried out with Effectene (Qiagen) or Cellfectin (Invitrogen) according to the manufacturers’ instructions. For exosome preparation, 7–40 ml of conditioned media obtained from cultures (1.25 × 106 cells / ml) were used as described19. The size and number of the exosomes in the P100 fraction were measured by Nanosight NC300 (Malvern Instruments).
Immunocytochemistry and Western analysis
Fly larvae were cultured at 25 °C unless stated otherwise. Wing discs from the late third instar larvae were used for intracellular staining and extracellular staining24. For immunocytochemistry, we used Sona-Pro, 1:300–500; Golgi (Calbiochem, mouse), 1:200; GFP (Abd serotec, sheep), 1:100; Senseless (a gift from H. Bellen, guinea pig), 1:1000; HRS (a gift from H. Bellen, guinea pig), 1:1000; WgN (sc-28646 Santa Cruz, rabbit), 1:100; Wg (DSHB, mouse), 1:1000; HA (Roche, rat), 1:300; HA (Santa Cruz, rabbit), 1:300; Vg (gift from Sean B. Carroll, rabbit), 1:100; Wg (DSHB, mouse), 1:100; Dll (Santa Cruz, goat), 1:100. For the extracellular staining of proteins, we used 10 times more antibodies than for the intracellular staining. Fluorescent images were captured using a Zeiss LSM laser scanning confocal microscope and processed with Adobe Photoshop.
Western analysis was carried out as described10. For western analysis, we used Sona-Pro (our lab, rabbit), 1:5000; HA (Santa Cruz, rabbit), 1: 250; GFP (Abcam, rabbit), 1:10,000; Wg (DSHB, mouse), 1:500–1000; Syntaxin 1A (DSHB, mouse), 1:25; Alix (gift from T. Aigaki, mouse), 1:500; Actin (DSHB, mouse), 1:500; Calnexin (gift from N.J. Colley, rabbit), 1:2000.
Electron microscopy
For immunogold labeling, P100 fraction from S2 sona-HA cells were plated on grids, blocked with 5% BSA in PBS and incubated with anti-HA antibody (1:5). Then, samples were washed with 0.1% BSA in PBS and incubated in secondary anti-rabbit antibody conjugated with 15 nm gold particles (AURION). After 8 times wash with PBS for 5 min each, samples were incubated in 1% glutaraldehyde for 5 min. Then, samples were washed with H2O for 8 times before staining with Phosphotungstic acid (PTA). Sample grids were air-dried completely and visualized using a transmission electron microscope (Talos F200X).
In vitro GST-Wg cleavage assay
For purification of GST-Wg, pGEX-4T-1-WgCterm was expressed in BL21 E. coli strain. Then, we purified the GST-Wg protein by standard column-based protocols (GST-column, 1st SP Sepharose column, 2nd SP Sepharose column). For purification of active Sona from S2 cell culture, CX and SNΔ fractions were prepared and lysis buffer without EDTA and Protein inhibitor cocktail (PIC) were added to these fractions. Active Sona was obtained by mixing with HA-conjugated bead and precipitating the beads. GST-Wg and active Sona were mixed and incubated at 25 °C overnight.
Sucrose step gradient
Exosome pellets were resuspended in 0.25 M sucrose and loaded on top of a sucrose step gradient before being centrifuged at 100,000xg in a Beckman SW41Ti rotor for 3 h as described77. Ten to twelve fractions of 1 mL each were then manually collected from the bottom of the gradient.
Wg reporter assay
The Wg reporter assay was carried out by conventional methods. WISIR vector that contains both firefly luciferase under the control of a Wg-responsive promoter and Renilla luciferase under the control of a Copia promoter was transfected into S2R+ cells. After one day of culture, cells were splitted to a 48 well plate and incubated for 3~4 h until the experimental treatment started. After 24 h of treatment, cells were lysed by following the manufacturer’s instructions of the Dual-Luciferase Repoter Assay System (Promega). Each condition was tested in triplicate.
References
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Apte, S. S. & Parks, W. C. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 44-46, 1–6 (2015).
Mott, J. D. & Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 16, 558–564 (2004).
Rocks, N. et al. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie 90, 369–379 (2008).
Porter, S., Clark, I. M., Kevorkian, L. & Edwards, D. R. The ADAMTS metalloproteinases. Biochem J. 386, 15–27 (2005).
Kelwick, R., Desanlis, I., Wheeler, G. N. & Edwards, D. R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 16, 113 (2015).
Le Goff, C. & Cormier-Daire, V. The ADAMTS(L) family and human genetic disorders. Hum. Mol. Genet. 20, 163–167 (2011).
El, HourM. et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene 29, 3025–3032 (2010).
Rocks, N. et al. ADAMTS-1 metalloproteinase promotes tumor development through the induction of a stromal reaction in vivo. Cancer Res. 68, 9541–9550 (2008).
Kim, G. W. et al. Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling. Sci. Rep. 6, 31863 (2016).
Banziger, C. et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509–522 (2006).
Bartscherer, K., Pelte, N., Ingelfinger, D. & Boutros, M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125, 523–533 (2006).
Tsogtbaatar, O. et al. An ADAMTS Sol narae is required for cell survival in Drosophila. Sci. Rep. 9, 1270 (2019).
Baldin, V., Lukas, J., Marcote, M. J., Pagano, M. & Draetta, G. Cyclin D1 Is a Nuclear-Protein Required for Cell-Cycle Progression in G(1). Gene Dev. 7, 812–821 (1993).
Sharma, R. P. Wingless—a new mutant in D. melanogaster. Drosoph. Inf. Serv. 50, 134 (1973).
Rijsewijk, F. et al. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 50, 649–657 (1987).
Mann, M. C. The occurrence and hereditary behavior of two new dominant mutations in an inbred strain of Drosophila melanogaster. Genetics 8, 27–36 (1923).
Port, F. & Basler, K. Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic 11, 1265–1271 (2010).
Gross, J. C., Chaudhary, V., Bartscherer, K. & Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 14, 1036–1045 (2012).
Gross, J. C. & Boutros, M. Secretion and extracellular space travel of Wnt proteins. Curr. Opin. Genet. Dev. 23, 385–390 (2013).
Bejsovec, A. Wingless/Wnt signaling in Drosophila: the pattern and the pathway. Mol. Reprod. Dev. 80, 882–894 (2013).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Delanoue, R. et al. The Drosophila wing differentiation factor Vestigial-Scalloped is required for cell proliferation and cell survival at the dorso-ventral boundary of the wing imaginal disc. Cell Death. Differ. 11, 110–122 (2004).
Strigini, M. & Cohen, S. M. Wingless gradient formation in the Drosophila wing. Curr. Biol. 10, 293–300 (2000).
Neumann, C. J. & Cohen, S. M. A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development 122, 3477–3485 (1996).
Ryoo, H. D., Gorenc, T. & Steller, H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the wingless signaling pathways. Dev. Cell 7, 491–501 (2004).
Angers, S. & Moon, R. T. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 (2009).
Shtutman, M. et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96, 5522–5527 (1996).
He, T. C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Koles, K. & Budnik, V. Wnt signaling in neuromuscular junction development. Cold Spring Harb. Perspect. Biol. 4, pii: a008045 (2012).
Herr, P., Hausmann, G. & Basler, K. WNT secretion and signalling in human disease. Trends Mol. Med. 18, 483–493 (2012).
Clevers, H. & Nusse, R. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 (2012).
Beckett, K. et al. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic 14, 82–96 (2013).
Koles, K. et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J. Biol. Chem. 287, 16820–16834 (2012).
Willert, K. & Nusse, R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 4, a007864 (2012).
Brook, W. J. & Cohen, S. M. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila Leg. Science 273, 1373–1377 (1996).
Port, F. et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat. Cell Biol. 10, 178–185 (2008).
Casares, F. & Mann, R. S. A dual role for homothorax in inhibiting wing blade development and specifying proximal wing identities in Drosophila. Developmen 127, 1499–1508 (2000).
Phillips, R. G. & Whittle, J. R. wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development 118, 427–438 (1993).
Couso, J. P., Bishop, S. A. & Martinez Arias, A. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120, 621–636 (1994).
Baena-Lopez, L. A., Alexandre, C., Mitchell, A., Pasakarnis, L. & Vincent, J. P. Accelerated homologous recombination and subsequent genome modification in Drosophila. Development 140, 4818–4825 (2013).
von Maltzahn, J., Zinoviev, R., Chang, N. C., Bentzinger, C. F. & Rudnicki, M. A. A truncated Wnt7a retains full biological activity in skeletal muscle. Nat. Commun. 4, 2869 (2013).
Yun, C. & Dasgupta, R. Luciferase reporter assay in Drosophila and mammalian tissue culture cells. Curr. Protoc. Chem. Biol. 6, 7–23 (2014).
Baena-Lopez, L. A., Franch-Marro, X. & Vincent, J. P. Wingless promotes proliferative growth in a gradient-independent manner. Sci. Signal. 2, 60 (2009).
Ren, F. et al. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc. Natl. Acad. Sci. USA 107, 21064–21069 (2010).
Cordero, J. B., Stefanatos, R. K., Myant, K., Vidal, M. & Sansom, O. J. Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development 139, 4524–4535 (2012).
Johnston, L. A. & Edgar, B. A. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature 394, 82–84 (1998).
Singh, A., Shi, X. & Choi., K. W. Lobe and Serrate are required for cell survival during early eye development in Drosophila. Development 133, 4771–4781 (2006).
Zhang, S. et al. The canonical Wg signaling modulates Bsk-mediated cell death in Drosophila. Cell Death Dis. 6, e1713 (2015).
Wu, C. H. & Nusse, R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J. Biol. Chem. 277, 41762–41769 (2002).
Diaz-Benjumea, F. J. & Cohen, S. M. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121, 4215–4225 (1995).
Maves, L. & Schubiger, G. A molecular basis for transdetermination in Drosophila imaginal discs: interactions between wingless and decapentaplegic signaling. Development 125, 115–124 (1998).
Wu, J. & Cohen, S. M. Proximodistal axis formation in the Drosophila leg: subdivision into proximal and distal domains by Homothorax and Distal-less. Development 126, 109–117 (1999).
Neumann, C. J. & Cohen, S. M. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124, 871–880 (1997).
Baeg, G. H., Selva, E. M., Goodman, R. M., Dasgupta, R. & Perrimon, N. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev. Biol. 276, 89–100 (2004).
Parker, D. S., Jemison, J. & Cadigan, K. M. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129, 2565–2576 (2002).
Nolo, R., Abbott, L. A. & Bellen, H. J. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349–362 (2000).
Lunde, K. et al. Activation of the knirps locus links patterning to morphogenesis of the second wing vein in Drosophila. Development 130, 235–248 (2003).
Jafar-Nejad, H., Tien, A. C., Acar, M. & Bellen, H. J. Senseless and Daughterless confer neuronal identity to epithelial cells in the Drosophila wing margin. Development 133, 1683–1692 (2006).
Zhang, X. et al. Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell 149, 1565–1577 (2012).
Datar, S. A., Jacobs, H. W., de la Cruz, A. F., Lehner, C. F. & Edgar, B. A. The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J. 19, 4543–4554 (2000).
Bouge, A. L. & Parmentier, M. L. Tau excess impairs mitosis and kinesin-5 function, leading to aneuploidy and cell death. Dis. Model Mech. 9, 307–319 (2016).
Chang, Y. H. & Sun, Y. H. Carrier of Wingless (Cow), a secreted heparan sulfate proteoglycan, promotes extracellular transport of Wingless. PLoS ONE 9, e111573 (2014).
Baeg, G. H., Lin, X., Khare, N., Baumgartner, S. & Perrimon, N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 128, 87–94 (2001).
Baena-Lopez, L. A. & Garcia-Bellido, A. Control of growth and positional information by the graded vestigial expression pattern in the wing of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 103, 13734–13739 (2006).
Janda, C. Y., Waghray, D., Levin, A. M., Thomas, C. & Garcia, K. C. Structural basis of Wnt recognition by Frizzled. Science 337, 59–64 (2012).
Bazan, J. F., Janda, C. Y. & Garcia, K. C. Structural architecture and functional evolution of Wnts. Dev. Cell. 23, 227–232 (2012).
Tang, X. et al. Roles of N-glycosylation and lipidation in Wg secretion and signaling. Dev. Biol. 364, 32–41 (2012).
Bejsovec, A. & Wieschaus, E. Signaling activities of the Drosophila wingless gene are separately mutable and appear to be transduced at the cell surface. Genetics 139, 309–320 (1995).
Menck, K. et al. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget 4, 2057–2066 (2013).
Luga, V. et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151, 1542–1556 (2012).
Panakova, D., Sprong, H., Marois, E., Thiele, C. & Eaton, S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435, 58–65 (2005).
Pfeiffer, S., Ricardo, S., Manneville, J. B., Alexandre, C. & Vincent, J. P. Producing cells retain and recycle Wingless in Drosophila embryos. Curr. Biol. 12, 957–962 (2002).
Pulipparacharuvil, S. et al. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 118, 3663–3673 (2005).
Giraldez, A. J., Copley, R. R. & Cohen, S. M. HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev. Cell. 2, 667–676 (2002).
Croker, J. A., Ziegenhorn, S. L. & Holmgren, R. A. Regulation of the Drosophila transcription factor, Cubitus interruptus, by two conserved domains. Dev. Biol. 291, 368–381 (2006).
Yan, R., Han, P., Miao, H., Greengard, P. & Xu, H. The transmembrane domain of the Alzheimer’s beta-secretase (BACE1) determines its late Golgi localization and access to beta -amyloid precursor protein (APP) substrate. J. Biol. Chem. 276, 36788–36796 (2001).
Schneider, I. Cell Lines Derived from Late Embryonic Stages of Drosophila-Melanogaster. J. Embryol. Exp. Morph. 27, 353 (1972).
Chu, M. L. et al. structural Studies of Wnts and identification of an LRP6 binding site. Structure 21, 1235–1242 (2013).
Acknowledgements
We thank K.W. Choi and K.H. Kang for their critical reading of this manuscript. We also thank Munashingha P. R. for preparation of GST-Wg protein. We are indebted to J. P. Vincent, K. Basler, S. Eaton, S. Hayashi, S. M. Cohen, L. S. Shashidhara, R. Holmgren, and H. Kramer for fly lines, to K. Basler, J. P. Vincent, and S.T. Hong for DNA constructs, and to H. Bellen, N.J. Colley, T. Aigaki and Sean B. Carroll for antibodies. We thank Bloomington Stock Center, Drosophila Genetic Resource Center, and Developmental Studies Hybridoma Bank for fly strains and antibodies. This research was supported by grants from the National Research Foundation of Korea (NRF-2017R1A2B4009254 and NRF-2019R1H1A2039726) and National Research Council of Science and Technology (DRC-14-KRISS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by E. Baehrecke
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Won, JH., Kim, GW., Kim, JY. et al. ADAMTS Sol narae cleaves extracellular Wingless to generate a novel active form that regulates cell proliferation in Drosophila. Cell Death Dis 10, 564 (2019). https://doi.org/10.1038/s41419-019-1794-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-1794-8
This article is cited by
-
Wg secreted by conventional Golgi transport diffuses and forms Wg gradient whereas Wg tethered to extracellular vesicles do not diffuse
Cell Death & Differentiation (2021)
-
Exosomal arrow (Arr)/lipoprotein receptor protein 6 (LRP6) in Drosophila melanogaster increases the extracellular level of Sol narae (Sona) in a Wnt-independent manner
Cell Death & Disease (2020)
-
POU domain motif3 (Pdm3) induces wingless (wg) transcription and is essential for development of larval neuromuscular junctions in Drosophila
Scientific Reports (2020)