Abstact
Conventional therapies and novel molecular targeted therapies against breast cancer have gained great advances over the past two decades. However, poor prognosis and low survival rate are far from expectation for improvement, particularly in patients with triple negative breast cancer (TNBC). Here, we found that lncRNA DANCR was significantly overregulated in TNBC tissues and cell lines compared with normal breast tissues or other type of breast cancer. Knockdown of DANCR suppressed TNBC proliferation both in vitro and in vivo. Further study of underlying mechanisms demonstrated that DANCR bound with RXRA and increased its serine 49/78 phosphorylation via GSK3β, resulting in activating PIK3CA transcription, and subsequently enhanced PI3K/AKT signaling and TNBC tumorigenesis. Taken together, Our findings identified DANCR as an pro-oncogene and uncoverd a new working pattern of lncRNA to mediate TNBC tumorigenesis, which may be a potential therapeutic target for improving treatment of TNBC.
Similar content being viewed by others
Introduction
Breast cancer is the most prevalent malignant tumor in women, and the efficacy of currently available therapies seems far from satisfactory, which severely threats the health of females from all over the world1,2. Generally, breast cancer can be classified into four subtypes, including luminal type A, luminal type B, human epidermal growth factor receptor-2 (HER2) positive type, and triple-negative breast cancer (TNBC) type3. TNBC demonstrates a lack of ER, PR, and HER2 expression by immunohistochemical results, and acts as a highly invasive subtype comprising about 20% of all breast cancer patients4,5. Furthermore, TNBC can exhibit high invasiveness, metastasis, high recurrence risk, and mortality rates, resulting in poor prognosis6. The lack of molecular targeted therapies and the poor survival of TNBC patients have fostered great endeavors to discover precise molecular targets for clinical treatment strategies. A growing body of evidence suggests that long non-coding RNAs (LncRNAs) are involved in TNBC progression through regulating tumor-related gene expression7,8,9. However, the precise molecular mechanisms by which lncRNA mediates TNBC progression remain unclear.
Differentiation antagonizing non-protein coding RNA (DANCR), an lncRNA encoded on human chromosome 4q12, has been identified as an oncogene in multiple malignant tumors, including colon cancer10, esophageal cancer11, hepatocellular carcinoma12, osteosarcoma13, TNBC14. DANCR was previously demonstrated to contribute to suppression of cell differentiation due to acting as a negative regulator15,16. Recent studies demonstrated that DANCR directly interacts with miR-758-3p17 and miR-57718 in non-small cell lung cancer and colorectal cancer, respectively. DANCR represses the expression of TIMP2/3 through physical binding with EZH2 in prostate cancer19. DANCR is also shown as a direct target of MYC in cancer20. Furthermore, DANCR expression is correlated with survival and/or prognosis of patients with hepatocellular carcinoma12, gastric cancer21, and colorectal cancer10. However, due to the molecular and phenotypic heterogeneity within and between different tumor types, mechanism explorations are required to elucidate the precise biological behaviour of DANCR in tumors, especially TNBC.
Here, we showed that DANCR is more highly expressed in TNBC compared with that in normal breast tissues, which exhibits poor prognosis. DANCR promoted proliferation and tumorigenesis in TNBC through activating ser49/78 phosphorylation of RXRA, and thus promoting PIK3CA expression. We also showed that signaling axis DANCR-RXRA-PI3K/AKT plays important roles in TNBC proliferation and tumorigenesis in vitro and vivo, respectively. In conclusion, the present study elucidated the function of DANCR in TNBC and might provide a novel of signaling pathway in the treatment of TNBC.
Materials and methods
Clinical samples
Between August 2013 and August 2015, clinical specimens containing breast cancer tissues including 60 triple-negative (TNBC) type, 15 HER2 type, 15 Luminal A type, and 15 Luminal B type, and 10 normal breast tissues were obtained from department of Breast Surgery in Meizhou People’ s Hospital. All these patients had not received chemotherapy and radiotherapy before the operation and all clinical samples were confirmed by pathology. This study protocol approval from the Research Ethics Committee of the Meizhou People’ s Hospital, and written informed consent from each participant were obtained.
Cell lines
BT549, MCF7, T47D, MDA-MB-231, MDA-MB-453, and MDA-MB-468 cells were purchased from Cell Bank of the Chinese Scientific Academy (Shanghai, China), and were cultured in Dulbecco’ s modified Eagle’ s medium (DMEM) (Hyclone, Life Technologies, CA) with 10% fetal bovine serum (Hyclone, Life Technologies, CA). MCF10A was also from Cell Bank of the Chinese Scientific Academy (Shanghai, China), and was cultured in DMEM/F12 (Hyclone, Life Technologies, CA) supplemented with 5% horse serum (Invitrogen, Carlsbad, CA), 20 ng/ml hEGF (Sigma-Aldrich), 0.5 μg/ml hydrocortisone (Sigma-Aldrich), 100 ng/ml cholera toxin (Sigma-Aldrich),10 μg/ml insulin (Sigma-Aldrich), and 100 U/ml penicillin-streptomycin (Sigma-Aldrich). BT549, MCF7, T47D, MDA-MB-231, MDA-MB-453, MDA-MB-468, and MCF10A were recently authenticated through using STR DNA fingerprinting from Shanghai Biowing Applied Biotechnology Co., Ltd. Moreover, by using LookOut Mycoplasma PCR Detection kit (Sigma-Aldrich), we detected the mycoplasma infection.
RNA extraction and quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and extracted according to the manufacturers’ protocol (Sigma-Aldrich). Reverse transcription (RT) was performed using the M-MLV Reverse Transcription Kit(Thermo Fisher Scientific). Quantitative RT–PCR was performed by means of Power qPCR Premix (SYBR Green) (Shang hai Generay Biotech CoCo., Ltd). GAPDH was used as a control. Primers are contained in Supplenentary Table S1.
Western blot analysis
Western blot was performed as we previously described22. The specific antibodies for WB as follows: anti-RXRA (21218-1-AP, 1:1000, Proteintech), Phospho-RXRA (PA5-64630,1:1000, Invitrogen), GSK3β (ab65740, 1:1000, Abcam), Akt (#4685S, 1:1000, Cell Signaling Technology), Phospho-Akt (#4060S, 1:1000, Cell Signaling Technology), PIK3CA (MA5-17149,1:1000, Thermo Fisher), GAPDH (#5174S, 1:1000, Cell Signaling Technology).
ChIP-qPCR
ChIP assay was performed using EZ-Magna ChIP™ A/G Chromatin Immunoprecipitation Kit (Millipore-17-408) referring to the manufacturer’s protocol.
Input genomic DNA and the purified immunoprecipitated DNA were used for qRT-PCR. Primers are contained in Supplementary Table 1.
Luciferase promoter assay
pGL3-PIK3CA promoter wild or mutant type of the RXRA binding was performed co-transfection with or without RXRA using Lipofectamine RNAiMAX Reagent (Thero Fisher) referring to the manufacturer’s protocol. pRL Renilla luciferase control reporter vector (Promega) was presented as a control. A dual-luciferase assay was analyzed 48 h after co-transfection using the Promega E1960 Dual-Luciferase® Reporter System in accordance with the manufacturer recommendation.
RNA immunoprecipitation (RIP) and RNA pull-down assays
The EZ-Magna RIP Kit (17-701, Millipore) was used for RIP assay with 10 μg anti-RXRA (21218-1-AP, Proteintech) referring to the manufacturer recommendation. The purified immunoprecipitated RNA and input genomic RNA were detected by quantitative RT-PCR. Biotin-labeled RNA was transcribed with Biotin RNA Labeling Mix (Roche 11685597910) and T7 RNA polymerase (Roche 10881775001), mixed with DNase I recombinant (Roche 04716728001), and purified with RNeasy Mini Kit (Qiagen 74904). Cell nuclear proteins were extracted using the pierce 78833 NE-PER (R) Nuclear and Cytoplasmic Extraction Reagents. Cell nuclear extract was mixed with Biotin-labeled RNA. Washed streptavidin agarose beads (Sigma-Aldrich) were added to each reaction. The binding protein was analyzed by Western blot assay.
Cell proliferation and soft agar colony formation
A WST-1 Assay Kit (Roche) was used for cell proliferation assay. Cells were seeded in a 48-well plate and then incubated at 37 °C. Cell numbers were assessed with the WST-1 Assay Kit. For soft agar colony formation, cells were were split into the suspension of a single-cell, and then seeded in a media containing 0.4% top layer agar and 0.8% bottom layer agar in a 6-well plate. Cell culture media was changed every 4 days after seeding. Colonies were fixed with 4% paraformaldehyde and stained with 2% crystal violet solution after 2–3 weeks, respectively. The visible colony numbers scored and data were analyzed.
Construction of vectors
The cDNA encoding DANCR, PIK3CA,GSK3β, RXRA were amplified from MCF10A cells and sequenced, and then subcloned into the pcDNA3 vector (Invitrogen), subsequently named pCDNA3-DANCR, pCDNA3-PIK3CA, pCDNA3-GSK3β, pCDNA3-RXRA. pLVX-DANCR, pLVX-PIK3CA, pLVX-GSK3β, and pLVX-RXRA was generated from pCDNA3-DANCR, pCDNA3-PIK3CA, pCDNA3-GSK3β, pCDNA3- RXRA, respectively. PIK3CA promoter was PCR-amplified from MCF10A cells and sequenced, and then subcloned into pGL3 vector (Promega). A Quik Change Site-Directed Mutagenesis Kit (Stratagene) was used for point mutations. shRNAs for DANCR were designed (shDANCR-1 target sequence: 5′-GGAGCTAG AGCAGTGACAATG-3′; shDANCR-2 target sequence 5′-GGTCACCAGACTTGCT ACACC-3′), and RXRA (shRXRA target sequence: 5′-GGCAAGCACTATGG AGTGTAC-3′, respectively.
Tumorigenesis studies
BALB/c-nude mice female at an age of 4–5 weeks (SLAC, Shanghai, China) were randomly divided into 4 per group, and then MDA-MB-231 cells (3 × 106) were implanted subcutaneously into mammary fat pads of each mice. All animal experiments procedures were approved by Zhejiang Provincial People’s Hospital the Guidance of Institutional Animal Care and Use Committee (IACUC). The IVIS Lumina imaging station (Caliper Life Sciences) was used for bioluminescence imaging. Different investigators independently performed mice allocation, surgery and the outcome assessing.
Statistical analysis
All statistical analyses were performed using the GraphPad Prism version 5.0. The significance of the data from patient specimens was determined by Pearson’ s correlation coefficient. The significance of data from the vitro and vivo between experimental groups was determined by the Student’ s test or Mann–Whitney U-test *P < 0.05 was considered statistically significant.
Results
Expression of DANCR in clinical TNBC specimens
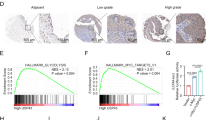
We examined DANCR expression using clinical samples consisting of TNBC tissues and normal breast tissues. DANCR was found to be significantly upregulated in TNBC tumor tissues compared with that in normal breast tissues (Fig. 1a). To support our finding, we downloaded the TCGA RNA-seq dataset and microarray dataset of invasive breast cancer specimens, respectively. In these datasets, There was a clear trend that the TNBC tissues exhibits higher DANCR expression compared to the paired peritumoral tissues (Fig. 1e and Supplementary Figure 1A). Interestingly, higher level of DANCR was demonstrated to be associated with bigger tumor size (Fig. 1b). We then detected DANCR expression in breast cancer samples of various subtypes. DANCR showed significantly higher expression level in TNBC than that in the other subtypes of breast cancer (Fig. 1c). Furthermore, patients with high DANCR expression (n = 30) suffered poorer overall survival (OS) as compared to low expression group (n = 30) (Fig. 1d). Similarly, TCGA microarray dataset also demonstrated the result (Supplementary Figure 1B). Gene amplifications in DANCR were observed in mRNA overexpression (TCGA database from cBioPortal) (Supplementary Figure 1C). Taken together, this data demonstrated that DANCR is amplified and overexpressed in TNBC tumors.
a Expression levels of DANCR mRNA are markedly higher in TNBC samples compared with normal breast tissues. b DANCR mRNA expression in clinical TNBC specimens with different tumor size groups (>5 cm and <5 cm). c Expression level of DANCR mRNA in breast cancers with TN (triple negative), Her2, Luminal A, and Luminal B types. d Kaplan–Meier analysis of patients with high DANCR mRNA-expressing TNBC versus low DANCR mRNA -expressing TNBC. Statistical analysis was performed by log-rank test in a GraphPad Prism version 5.0 for Windows. e DANCR mRNA expression levels are markedly higher in clinical TNBC samples as compared to paired peritumoral breast tissues. Data of DANCR mRNA expression were downloaded from the Cancer Genome Atlas (TCGA) RNA-seq dataset. Error bars ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. Data are representative from two independent experiments
DANCR inhibition suppressed cell proliferation and tumor growth in TNBC
To further demonstrate the involvement of DANCR in TNBC, we tested DANCR expression in six breast cancer cell lines (BT549, MCF7, T47D, MDA-MB-231, MDA-MB-453, and MDA-MB-468) and a normal mammary epithelial cell line (MCF10A). DANCR expression was markedly upregulated in breast cancer cell lines as compared to that in MCF10A cell lines (Fig. 2a). Importantly, among all the six cancer cell lines, DANCR exhibited highest expression levels in two TNBC cell lines (MDA-MB-231 and MDA-MB-468).
a qRT-PCR analysis of DANCR mRNA levels in breast normal and cancer cells. b qRT-PCR analysis of DANCR mRNA levels knockdown using two different shRNAs (shDANCR-1 and shDANCR-2) or a control shRNA (shC) in both MDA-MB-231 and MDA-MB-468 cells. c, d Effects of DANCR knockdown on TNBC cell proliferation (c) and soft agar colony formation (d). e Quantification of soft agar colony formation in D. f Representative bioluminescence images of shDANCR or shC-transfected MDA-MB-231 cells injected into the mice mammary gland fat pads. Mice were imaged at 4 weeks after transplantation. Data were from two independent experiments with 4 mice per group with similar results. g Quantification of bioluminescence activity in (f). Error bars ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. Data are representative from two independent experiments
We then tested the effect of DANCR inhibition on TNBC cell growth. Here, we knocked down the DANCR expression through treating MDA-MB-231 and MDA-MB-468 cells with the short hairpin RNA-mediated DANCR silencing (shRNA) or non-silencing control (shC), and found that depletion of endogenous DANCR significantly suppressed cell proliferation in both TNBC cell lines compared with the controls (Fig. 2b, c). Moreover, DANCR knockdown also impaired soft agar colony formation in MDA-MB-231 and MDA-MB-468 cells (Fig. 2d, e).
To further elucidate the TNBC tumorigenesis with DANCR, analysis of the orthotopic breast cancer model was performed. MDA-MB-231 cell lines transduced with shDANCR-1, shDANCR-2 or shControl (shC) were separately injected into the mice mammary gland fat pads. Remarkably, knockdown of DANCR was significantly decreased TNBC tumor growth compared with controls (Fig. 2f, g). These data suggested that DANCR contributes to TNBC cell proliferation and tumor growth.
DANCR interacts with RXRA in TNBC cells
Recent studies have demonstrated that lncRNAs mainly function as sponges to bind functional proteins and then influence their downstream genes expression23,24,25. Thus, We hypothesized that DANCR-regulated tumorigenesis depends on its binding proteins. DANCR was examined for transcription factor binding sites using a JASPAR database of transcription factor binding profiles, which identified two RXRA-binding sites as the most potential candidates. It is predicted that RXRA may bind with DANCR at both 211 to 225 and 269 to 283 sites (Fig. 3a). To test this, we performed RIP quantitative PCR with an antibody against RXRA from nuclear extracts of both MDA-MB-231 and MDA-MB-468 cells. We demonstrated that DANCR specifically bound to endogenous RXRA protein (Fig. 3b).
a Schematic diagram of putative RXRA binding sites in DANCR. b RIP-qPCR assay of the association of RXRA with DANCR in MDA-MB-231 and MDA-MB-468 cells. c Re-expression of shRNA-resistant DANCR wild type and RXRA-binding mutant types. d RIP-qPCR assay of effects of re-expression of shRNA-resistant DANCR wild type or mutant types on RXRA binding. e Biotinylated DANCR was incubated with nuclear extracts (MDA-MB-231 and MDA-MB-468 cells), targeted with streptavidin beads, and binding proteins were resolved in a gel. Western blotting assay of the specific binding of RXRA and DANCR. f, g RNAs corresponding to fragments in different regions of DANCR were treated as in (e), and binding RXRA was detected by western blotting assay. Error bars ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. Data are representative from two independent experiments
We next constructed DANCR plasmids containing a wild type and three mutant types including DANCR-RXRA binding site1 (Mut1), site2 (Mut2), or both sites (Mut1/2). As we predicted, re-expression of shRNA-resistant DANCR cDNA encoding the wild type restored the binding of DANCR with RXRA, whereas re-expression of a DANCR shRNA-resistant plasmid containing the DANCR-RXRA binding site1, site 2, or two sites mutants did not rescue it, suggesting that these two sites are critical for DANCR-RXRA binding (Fig. 3c, d).
To further validate the binding between DANCR and RXRA, we performed RNA pulldown (Fig. 3e), and deletion-mapping methods (Fig. 3f, g) to demonstrate whether RXRA would bind within specific regions of DANCR. These data identified a 300nt region at the 5′ end of DANCR required for the binding with RXRA (Fig. 3g). Taken together, the RIP, RNA pulldown, and deletion-mapping data validate a specific binding between RXRA and DANCR.
DANCR regulates RXRA phosphorylation
To explore the role of RXRA in DANCR-mediated TNBC tumor growth, we first assessed RXRA protein and expression in DANCR depletion TNBC cells. We found that DANCR knockdown did not affect the protein level of RXRA in MDA-MB-231 and MDA-MB-468 cells (Fig. 4a). Moreover, DANCR depletion had no effect on RXRA mRNA level (Fig. 4b). However, Western blot analysis showed that lower phosphorylation level of RXRA was found in the DANCR-knockdown group as compared to that of the controls (Fig. 4c). Furthermore, re-expression of shRNA resistant DANCR wild type rescued DANCR knockdown-inhibited RXRA phosphorylation, whereas re-expression of shRNA resistant DANCR mutant type of the RXRA binding did not affect it (Fig. 4d). Together, Our data suggest that DANCR enhance RXRA protein phosphorylation in TNBC.
DANCR facilitates PIK3CA transcription in a RXRA-mediated manner
Since PI3K signaling is critical for TNBC proliferation and Akt is a dominant downstream effector of PI3K signaling pathway26, we explored the effects of DANCR depletion on expression of phosphatidylinositol-4,5-biophosphate 3-kinase catalytic subunit alpha (PIK3CA) in MDA-MB-231 and MDA-MB-468 cells. As shown in Fig. 5a, b, knockdown of DANCR significantly inhibited PIK3CA protein and mRNA expression levels in both TNBC cells. However, knockdown of RXRA significantly rescued DANCR depletion-inhibited PIK3CA expression (Fig. 5c). These data suggest that DANCR may mediates RXRA to regulate PIK3CA expression.
a, b Effects of DANCR knockdown on PIK3CA protein (a) and mRNA (b) expression in MDA-MB-231 and MDA-MB-468 cells. c Knockdown of RXRA restores DANCR depletion-inhibited PIK3CA expression in MDA-MB-231 and MDA-MB-468 cells. d Schematic diagram of the putative RXRA binding site in PIK3CA promoter. e ChIP-qPCR assay of the binding of RXRA with the PIK3CA promoter in MDA-MB-231 cells. f Luciferase assay of RXRA mediation on the PIK3CA promoter activity in MDA-MB-231 cells. g Knockdown of RXRA and overexpression of PIK3CA in DANCR depletion cells in MDA-MB-231 cells. h, i Knockdown of RXRA and overexpression of PIK3CA rescue DANCR knockdown-inhibited cell proliferation (h) and soft agar colony formation (i) in MDA-MB-231 cells. Error bars ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. Data are representative from two independent experiments
To validate that RXRA regulates PIK3CA expression in TNBC cells, we predicted RXRA-binding sites to the promoter of PIK3CA using the JASPAR database of transcription factor binding profiles. One RXRA binding site was detected in the promoter of PIK3CA at the −1148 to −1134 site (Fig. 5d). RXRA Knockdown increased PIK3CA mRNA levels by qRT-PCR assays (Supplementary Figure 3). ChIP-qPCR assays using the antibody against RXRA demonstrated that RXRA bind to PIK3CA promoter (Fig. 5e). Promoter luciferase assays further validated that RXRA overexpression markedly decreased PIK3CA promoter transcriptional activity compared with the control, whereas the transcriptional activity was definitely restored after the mutation of RXRA binding site in PIK3CA promoter(Fig. 5f). Taken together, these data indicate that RXRA acts as a transcription repressors to inhibit PIK3CA expression.
To validate that DANCR/RXRA/PIK3CA signaling pathway regulates TNBC tumor growth, we down-regulated RXRA and overexpressed PIK3CA in MDA-MB-231 and MDA-MB-468 cells with a RXRA shRNA or a PIK3CA vector (Fig. 5g and Supplementary Figure 2A). Knockdown of RXRA rescued DANCR depletion-inhibited PIK3CA and Akt phosphorylation (Fig. 5g and Supplementary Figure 2A), cell proliferation (Fig. 5h and Supplementary Figure 2B), colony formation (Fig. 5i and Supplementary Figure 2C). Moreover, overexpression of PIK3CA restored Akt phosphorylation (Fig. 5g and Supplementary Figure 2A), cell proliferation (Fig. 5h and Supplementary Figure 2B), colony formation (Fig. 5i and Supplementary Figure 2C) inhibited by DANCR knockdown. Furthermore, DANCR expression was found to be correlated with PIK3CA expression (Supplementary Figure 2D). These data further demonstrate that DANCR mediates RXRA to upregulate PIK3CA expression, resulting in enhancing PI3K/AKT signaling pathway and promoting TNBC tumor growth.
DANCR-mediated RXRA phosphorylation depends on GSK3β
Since the glycogen synthase kinase 3 beta (GSK3β) had been reported to promote RXRA phosphorylation in colorectal cancer cells27, we detected whether DANCR-regulated RXRA phosphorylation depends on GSK3β. As shown in Fig. 6a, compared with the control group, overexpression of DANCR increased RXRA binding with GSK3β, RXRA phosphorylation, Akt phosphorylation and PIK3CA expression in both TNBC cells (Fig. 6a). Knockdown of DANCR decreased RXRA binding with GSK3β, RXRA phosphorylation, Akt phosphorylation and PIK3CA expression in both TNBC cells (Fig. 6b). Furthermore, overexpression of GSK3β increased RXRA phosphorylation, Akt phosphorylation and PIK3CA expression (Fig. 6c) inhibited by DANCR knockdown. Consistent with previous study27, overexpression of RXRA increased its binding with GSK3β and decreased Akt phosphorylation and PIK3CA expression in MDA-MB-231 and MDA-MB-468 cells (Fig. 6d). Overexpression of RXRA significantly restored GSK3β association and Akt phosphorylation and PIK3CA expression inhibited by DANCR knockdown (Fig. 6d). These data support that DANCR depends on GSK3β to mediate RXRA phosphorylation in both TNBC cells.
a Effects of DANCR overexpression on RXRA-GSK3β binding and RXRA phosphorylation expression in MDA-MB-231 and MDA-MB-468 cells. b Effects of DANCR depletion on RXRA-GSK3β binding and RXRA phosphorylation expression. c GSK3β overexpression restored DANCR knockdown-inhibited RXRA phosphorylation. d Effects of overexpression of RXRA on RXRA-GSK3β binding in MDA-MB-231/shC and MDA-MB-231/shDANCR cells
Ser49/Ser78 sites of RXRA protein are critical for DANCR-mediated TNBC tumor growth
Since the roles of RXRA phosphorylation mediated by DANCR in TNBC is not well understood, we focused on the role of RXRA phosphorylation in DANCR-regulated TNBC cell proliferation. To detect whether RXRA phosphorylation is necessary to activate PI3K/AKT signaling pathway, we generated the GSK3β kinase deficient mutant plasmid, GSK3β-K58A. As shown Fig. 7a, overexpression of GSK3β cDNA encoding the wild type restored DANCR knockdown-inhibited RXRA phosphorylation, Akt phosphorylation and PIK3CA expression, whereas transfection of GSK3β kinase deficient mutant type did not rescue it. Next, we predicted potential serine phosphorylation sites of the RXRA protein using the Phosphor Motif Finder Program. Two serine phosphorylation candidate sites at Ser49 and Ser78 were identified. Transfection of RXRA wild type in MDA-MB-231 cells restored its binding with GSK3β inhibited by DANCR knockdown, and further decreased DANCR depletion-inhibited Akt phosphorylation, PIK3CA expression, cell proliferation, and soft agar colony formation (Fig. 7b–e). However, transfection of RXRAS49A/S78A mutant did not rescued it compared with the wild type group (Fig. 7b–e). Taken together, these data demonstrates that serine phosphorylation of RXRA plays a crucial role in activating the PI3K/AKT signaling pathway.
a Effects of overexpression of GSK3β wild type or kinase deficient mutant type on RXRA phosphorylation and PI3K/Akt signaling activity in MDA-MB-231/shC and MDA-MB-231/shDANCR cells. b Effects of overexpression of RXRA wild type or mutant type on RXRA-GSK3β binding on RXRA phosphorylation and PI3K/Akt signaling activity in MDA-MB-231 with shC and shDANCR cells. c–e Effects of overexpression of RXRA wild type or mutant type on RXRA-GSK3β binding on RXRA protein binding PIK3CA ability (c), cell proliferation (d), and soft agar colony formation (e). Error bars ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. Data are representative from two independent experiments
Discussion
In this study, we demonstrated a new mechanism by which DANCR-upregulated PI3K/AKT signaling pathway through activating serine phosphorylation of RXRA protein is important for TNBC cell proliferation and tumor growth (Fig. 8). By means of facilitating connection between RXRA and GSK3β, DANCR promotes the phosphorylation of RXRA, leading to suppress RXRA induced inhibition of PIK3CA transcription, thereby activating PI3K/AKT downstream signaling and ultimately promoting TNBC tumorigenesis.
Our data demonstrate that DANCR promotes TNBC tumorigenesis. DANCR was previously identified to play an important role in maintaining the undifferentiated cell state15,16. Previous work demonstrated that DANCR interacts with EZH2 to form a complex to promote stem cell characteristics16,28. It has been well documented that DANCR was overexpressed and amplified in breast cancer, and knockdown of DANCR significantly inhibited cell proliferation and invasion in breast cancer through facilitating binding of EZH2 to the promoters of ABCG2 and CD44 genes14. Here, we report that DANCR is upregulated in clinical TNBC samples, and higher DANCR level is positively correlated with poorer prognosis of TNBC patients. Knockdown of DANCR significantly inhibited TNBC cell proliferation, colony formation in vitro, and tumor growth in vivo. These results strongly support that DANCR is critical for TNBC tumorigenesis.
Our results also demonstrate that DANCR mediates TNBC through RXRA. RXRA had been reported to play critical roles in breast cancer cell progression29,30. Phosphorylation of the RXRA at serine 260 decreases its coactivator recruitment ability, leading to inhibition of RXRA transcriptional activity and enhanced cancer cell proliferation31,32. DANCR has also been identified as a molecular sponge mediating miR-758-3p17 and miR-57718 in non-small cell lung cancer and colorectal cancer, respectively. Furthermore, some studies showed that DANCR regulate breast cancer through binding and phosphorylating EZH225. In this study, our data showed that DANCR directly bound with RXRA and mediated its phosphorylation. Knockdown of DANCR enhanced RXRA phosphorylation. Re-expression of shRNA resistant DANCR wild type impaired DANCR knockdown-promoted RXRA phosphorylation, whereas re-expression of shRNA resistant mutant type of the DANCR-RXRA binding did not affect it. Knockdown of RXRA rescued DANCR depletion-inhibited cell proliferation and soft agar colony formation. Taken together, our data demonstrate that DANCR regulate TNBC cell proliferation through RXRA phosphorylation.
Our results further suggest that DANCR mediates TNBC through RXRA- downregulating PI3K/AKT signaling pathway. Activating PI3K/AKT signaling was reported to be associated with poor OS in patients with breast cancer33. PIK3CA activation was also demonstrated to be critical for enhancing PI3K/AKT signaling22. RXRA was reported to mediate PI3K/AKT signaling in response to stem cell differentiation and provoke tumor suppression34. Inhibition of the N-terminally truncated RXRA association with the PI3K p85α subunit also resulted in suppression of PI3K/AKT signaling activation35. Here, we found that RXRA binds with the promoter of PIK3CA and downregulate PIK3CA transcription. We also observed that knockdown of DANCR inhibited RXRA protein phosphorylation and PIK3CA expression level. Interestingly, knockdown of RXRA rescued DANCR depletion-inhibited PIK3CA expression, cell proliferation, soft agar colony formation, and the promoter transcriptional activity of PIK3CA. Furthermore, We found DANCR markedly promotes RXRA association with GSK3β. In consistent with the previous study31,32, our data demonstrate that DANCR-induced RXRA phosphorylation suppresses RXRA-inhibited PIK3CA transcription in TNBC cells and ultimately activates the downstream PI3K/AKT signaling, whereas overexpression of GSK3β mutant type with deficient kinase activity or RXRA with mutated binding site with GSK3β dramatically blocked it. These data suggest that DANCR promoted TNBC tumorigenesis depends on its binding and phosphorylating RXRA, which leads to PI3K/AKT signaling pathway activation.
In summary, our results identified DANCR as an oncogene promotes TNBC tumorigenesis through a distinctive mechanism by which DANCR facilitated RXRA phosphorylation depending on GSK3β, thereby inhibiting the function of RXRA as a transcription repressors, and ultimately enhanced downstream PI3K/AKT signaling. Our findings have shed light into a novel roles of DANCR in TNBC tumorigenesis which have significant implications on better understanding the function of DANCR in human cancers. We hope that the newly established roles of DANCR and RXRA in tumorigenesis may provide a strong rationale for targeting them to improve the treatment of TNBC patients.
References
Desantis, C., Ma, J., Bryan, L. & Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 64, 52–62 (2014).
Donepudi, M. S., Kondapalli, K., Amos, S. J. & Venkanteshan, P. Breast cancer statistics and markers. J. Cancer Res. Ther. 10, 506 (2014).
Weigelt, B., Eberle, C., Cowell, C. F., Ng, C. K. & Reisfilho, J. S. Metaplastic breast carcinoma: more than a special type. Nat. Rev. Cancer 14, 147–148 (2014).
Denkert, C., Liedtke, C., Tutt, A. & Von, M. G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 389, 2430 (2016).
Bianchini, G., Balko, J. M., Mayer, I. A., Sanders, M. E. & Gianni, L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 13, 674 (2016).
Lehmann, B. D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 121, 2750 (2011).
Li, W. et al. The FOXN3-NEAT1-SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J. Clin. Investig. 127, 3421–3440 (2017).
Zhou, W. et al. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200b/c and let-7b. Sci. Signal. 10, 483 (2017).
Lin, A. et al. The LINK-A lncRNA interacts with PtdIns(3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat. Cell Biol. 19, 238–251 (2017).
Liu, Y., Zhang, M., Liang, L., Li, J. & Chen, Y. X. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 8, 11480 (2015).
Shi, H. et al. Long non-coding RNA DANCR promotes cell proliferation, migration, invasion and resistance to apoptosis in esophageal cancer. J. Thorac. Dis. 10, 2573 (2018).
Yuan, S. X. et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma via de-repression of CTNNB1. Hepatology 63, 499–511 (2016).
Jiang, N. et al. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 405, 46–55 (2017).
Sha, S., Yuan, D., Liu, Y., Han, B. & Zhong, N. Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol. Open 6, 1310–1316 (2017).
Kretz, M. et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 26, 338 (2012).
Zhu, L. & Xu, P. C. Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem. Biophys. Res. Commun. 432, 612–617 (2013).
Wang, S. & Jiang, M. The long non-coding RNA-DANCR exerts oncogenic functions in non-small cell lung cancer via miR-758-3p. Biomed. Pharmacother. 103, 94–100 (2018).
Wang, Y. et al. Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp. Mol. Med. 50: 57 (2018).
Jia, J. et al. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget 7, 37868–37881 (2016).
Lu, Y. et al. MYC targeted long noncoding RNA DANCR promotes cancer in part by reducing p21 levels. Cancer Res. 78, 64–74 (2018).
Hao, Y. P., Qiu, J. H., Zhang, D. B. & Yu, C. G. Long non-coding RNA DANCR, a prognostic indicator, promotes cell growth and tumorigenicity in gastric cancer. Tumour Biol. 39, 1010428317699798 (2017).
Tang, J., Zhong, G., Wu, J., Chen, H. & Jia, Y. SOX2 recruits KLF4 to regulate nasopharyngeal carcinoma proliferation via PI3K/AKT signaling. Oncogenesis 7, 61 (2018).
Xu, M. D. et al. A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clin. Cancer Res. 23, 2071 (2016).
Wilusz, J. E., Sunwoo, H. & Spector, D. L. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 23, 1494–1504 (2009).
Z, L. et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 24, 59 (2016).
Hatem, R. et al. Targeting mTOR pathway inhibits tumor growth in different molecular subtypes of triple-negative breast cancers. Oncotarget 7, 48206–48219 (2016).
Liang, J. et al. miR-27a-3p targeting RXRα promotes colorectal cancer progression by activating Wnt/β-catenin pathway. Oncotarget 8, 82991–83008 (2017).
Jia, Q., Jiang, W. & Ni, L. Down-regulated non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of periodontal ligament stem cells. Arch. Oral. Biol. 60, 234–241 (2015).
Peng, X., Yun, D. & Christov, K. Breast cancer progression in MCF10A series of cell lines is associated with alterations in retinoic acid and retinoid X receptors and with differential response to retinoids. Int. J. Oncol. 25, 961–971 (2004).
Sun, S. Y. & Lotan, R. Retinoids and their receptors in cancer development and chemoprevention. Crit. Rev. Oncol. Hematol. 41, 41–55 (2002).
Matsushima-Nishiwaki, R. et al. Phosphorylation of retinoid X receptor alpha at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Res. 61, 7675 (2001).
Macoritto, M. et al. Phosphorylation of the human retinoid X receptor alpha at serine 260 impairs coactivator(s) recruitment and induces hormone resistance to multiple ligands. J. Biol. Chem. 283, 4943–4956 (2008).
Qiu, Z., Li, Y., Zeng, B., Guan, X. & Li, H. Downregulated CDKN1C/p57kip2 drives tumorigenesis and associates with poor overall survival in breast cancer. Biochem. Biophys. Res. Commun. 497, 187 (2018).
Zhang, R. et al. RXRα provokes tumor suppression through p53/p21/p16 and PI3K-AKT signaling pathways during stem cell differentiation and in cancer cells. Cell Death Dis. 9, 532 (2018).
Chen, L. et al. Sulindac-derived RXRα modulators inhibit cancer cell growth by binding to a novel site. Chem. Biol. 21, 596–607 (2014).
Acknowledgements
This study was supported in part by grants from the Zhejiang Provincial Nature Science Foundation of China (Grant number: LY18H160035 to Xiaodong Liang).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by B. Rotblat
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, J., Zhong, G., Zhang, H. et al. LncRNA DANCR upregulates PI3K/AKT signaling through activating serine phosphorylation of RXRA. Cell Death Dis 9, 1167 (2018). https://doi.org/10.1038/s41419-018-1220-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-018-1220-7
This article is cited by
-
A review on the role of DANCR in the carcinogenesis
Cancer Cell International (2022)
-
LncRNAs in breast cancer: a link to future approaches
Cancer Gene Therapy (2022)
-
MYBL2-induced PITPNA-AS1 upregulates SIK2 to exert oncogenic function in triple-negative breast cancer through miR-520d-5p and DDX54
Journal of Translational Medicine (2021)
-
Heparin-binding growth factor (HDGF) drives radioresistance in breast cancer by activating the STAT3 signaling pathway
Journal of Translational Medicine (2021)
-
Long noncoding RNAs: fine-tuners hidden in the cancer signaling network
Cell Death Discovery (2021)