Abstract
Direct reversion of cancers into normal-like tissues is an ideal strategy for cancer treatment. Recent reports have showed that defined transcription factors can induce reprogramming of cancer cells into pluripotent stem cells, supporting this notion. Here, we have developed a reprogramming method that uses a conceptually unique strategy for breast cancer cell treatment. We have screened a kinase inhibitor library and found that Rho-associated protein kinase (ROCK) and mammalian target of rapamycin (mTOR) kinase inhibitors can substitute for all transcription factors to be sufficient to reprogram breast cancer cells into progenitor cells. Furthermore, ROCK–mTOR inhibitors could reprogram breast cancer cells to another terminal lineage-adipogenic cells. Genome-wide transcriptional analysis shows that the induced fat-like cells have a profile different from breast cancer cells and similar to that of normal adipocytes. In vitro and in vivo tumorigenesis assays have shown that induced fat-like cells lose proliferation and tumorigenicity. Moreover, reprogramming treatment with ROCK–mTOR inhibitors prevents breast cancer local recurrence in mice. Currently, ROCK–mTOR inhibitors are already used as antitumor drugs in patients, thus, this reprogramming strategy has significant potential to move rapidly toward clinical trials for breast cancer treatment.
Similar content being viewed by others
Introduction
Reprogramming healthy somatic cells into pluripotent stem cells (iPSCs) with defined factors have been intensively investigated1,2,3. However, reprogramming cancer cells have fallen much behind4,5,6. Reprogramming and oncogenic transformation are stepwise processes that share many similarities. There are the classic reports of transplanting tumor cells into embryonic tissues, showing that the niche has an influence on tumorigenic behavior. Although unidentified biological barriers may exist6,7,8, reprogramming of both solid and liquid tumors to iPSCs has been reported by different groups7,9,10,11,12,13,14,15,16,17,18. Loss of tumorigenicity by unknown mechanisms and induced dedifferentiation to pluriopotency seem to be common features of reprogrammed cells from different cancers. However, robust differentiation into specific lineages remains a stumbling block2,3,19,20,21,22. We and others found that tumor-suppressor genes are a roadblock for both cellular reprogramming and oncogenic transformation6,7,8,23,24. Based on these results, we hypothesize that cancer cells could be reprogrammed into normal-like cells under the defined reprogramming conditions.
Integration-free reprogramming of cancer cells would be safer and preferable for clinical use. Along those lines, we screened a kinase inhibitor library and found that a combination of the inhibitors for two kinases, Rho-associated protein kinase (ROCK) and mammalian target of rapamycin (mTOR), can reprogram human breast cancer cells into progenitor cells. We can also trans-differentiate breast cancer cells into another terminal lineage-adipogenic (fat-like) cell. These cells lost tumorigenicity and came back to a “normal state”. Importantly, ROCK–mTOR inhibitor reprogramming treatment prevented breast cancer local recurrence in mice, while ROCK–mTOR inhibitor treatment without reprogramming condition only showed a limited effect on breast cancer recurrence. This indicates that reprogramming treatment plays a key role in preventing breast cancer recurrence.
Results
Screening of a protein kinase inhibitor library to reprogram breast cancer cells
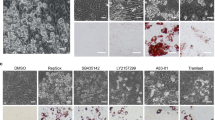
While somatic cells are reprogrammed to iPSCs by expression of transcription factors, it may cause genomic instability that increases the risk of cancer cell induction25,26,27,28,29. Therefore, we tried to develop a transgene-free method to efficiently reprogram breast cancer cells. Cellular senescence has been shown to regulate reprogramming of fibroblasts to iPSCs and fibroblast–neuron conversion23,24,30,31. Since many protein kinases are involved in senescence and proliferation processes, we screened a protein kinase inhibitor library (355 inhibitors, Calbiochem). We prepared a breast cancer cell line (MDA-MB-468) with expression of Nanog promoter-RFP, a progenitor marker protein. Through phenotypic change screening, we found that candidate kinase inhibitors reprogrammed breast cancer cells to induced progenitor-like cells (iPLs) in induction medium (Fig. 1a). After 7 days in induction medium with candidate kinase inhibitor treatment, we observed that a subpopulation of cells became Nanog-RFP positive with a marked morphological change. These ranged from large nuclear and flat-shaped cells (cancer cells) to small, bi- or multi-polar cells, termed iPLs (Fig. 1a). We confirmed that two candidate small molecules, namely rapamycin (mTOR inhibitor) and Y27632 (ROCK inhibitor), induced morphological change and RFP-positive staining with high efficacy (~30–50% efficacy, Fig. 1b). To further determine the combinational effects of these inhibitors on breast cancer cell conversion, we found that using mTOR–ROCK inhibitors (Rapamycin/Y27632) converted breast cancer cells into iPLs with ~90% efficacy after 7 days of induction (Fig. 1b).
a Screening design. Human breast cancer cells (MDA-MB-468) with expression of Nanog-promoter-RFP were seeded into 96-well plates. Kinase inhibitors from a library (Calbiochem) were added at a final concentration of 2 µM in the induction medium. The medium was changed every other day until day 7, when cells converted to RFP-positive cells. Essential hits were identified by RFP-positive cells as iPLs. Images were taken on day 7 after inhibitor treatment. Positive iPLs were counted by RFP-positive staining and quantified on day 7. b Screening results. MDA-MB-468 cells were treated with candidate kinase inhibitors. R + Y: Rapamycin + Y27632. Quantitative data are the mean ± SEM from three independent experiments. c Expression of progenitor markers and pluripotent markers during breast cancer cell reprogramming by mTOR–ROCK inhibitor treatment. qRT-PCR assays were performed for the mRNA expression of indicated markers in the MDA-MB-468 cells following mTOR + ROCK (R + Y: Rapamycin + Y27632) inhibitor treatment
Next, we analyzed the expression of progenitor markers as well as reprogramming markers in iPLs and parental breast cancer cells. qRT-PCR data showed that a panel of the markers, SOX-2, Nanog, Nestin, and Pax-6, are upregulated and expressed at day 3 and day 7 during the reprogramming process (Fig. 1c), confirming that there might exist a progenitor stage for the induced cells.
Reprogrammed breast cancer cells into mature connective tissue phenotypes
We next assessed whether cancer cells can be terminally reprogrammed and differentiated into another lineage (adipocytes, a lineage switch) in adipocyte induction medium. As terminal differentiation is defined as expression of the mature phenotype and cessation of proliferation, we assessed these properties in reprogrammed cells. As seen in Fig. 2, breast cancer cells (MDA-MB-468) with the presence of ROCK–mTOR inhibitor in adipocyte induction medium expressed the terminal phenotype of that differentiation lineage. Fat formation in induced cells was measured by lipid accumulation via Oil-Red-O, considered “gold standards”32,33 for terminal differentiation of embryonic and mesenchymal stem cells into adipocytes. In 2–3 weeks, 80–90% of breast cancer cells were induced to fat-like cells (iFLs) with mature adipocyte morphology that showed Oil-Red-O positive staining (Fig. 2a). In contrast, after 3 weeks in induction medium without kinase inhibitors, control breast cancer cells maintained cancer cell morphology with negative staining of Oil-Red-O (Fig. 2b).
a MDA-MB-468 cells were induced by ROCK–mTOR inhibitors in adipocyte induction medium at indicated time points. Scale bar, 10 µm. b Kinetic analysis of iFLs from MDA-MB-468 cells was performed after induction with ROCK–mTOR inhibitors. Quantitation of induced cells was counted by Oil-Red-O positive staining. Quantitative data are the mean ± SEM from three independent experiments. c Comparison of breast cancer cells and iFLs via Oil-Red-O staining for lipid accumulation. Scale bar, 10 µm. d 4T1 and HCC2157 cells were induced by ROCK–mTOR inhibitors in induction medium at indicated time points. Scale bar, 10 µm. e Positive iFLs from 4T1 and HCC2157 were counted by Oil-Red-O positive staining. Quantitative data are the mean ± SEM from three independent experiments
To investigate whether ROCK–mTOR inhibitors have a general effect on human breast cancer cells, the reprogramming treatment of ROCK–mTOR inhibitors was used in another two breast cancer cell lines, HCC2157 and 4T1. After induction for 3 weeks, about 80–90% of breast cancer cells were converted into iFLs (Fig. 2d, e). These results suggest that ROCK–mTOR inhibitor treatment led to conversion of breast cancer cells to fat-like cells.
Genome-wide transcriptional profiling of induced adipocytes
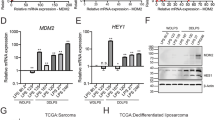
To analyze the similarities between iFLs and parental breast cancer cells, we generated comparative global gene expression data by microarray analysis. Microarray data were quantile normalized and filtered based on the average signal, and differently expressed genes were selected for further analysis. Hierarchical cluster analysis revealed a significant difference between iFLs and their parental breast cancer MDA-MB-468 cells (Fig. 3a, b, p < 0.001). Furthermore, the clustering analysis of global gene expression revealed that iFLs from MDA-MB-468 cells by the reprogramming treatment of ROCK–mTOR inhibitors were particularly similar to the control normal adipocytes (Fig. 3b, p > 0.5). A panel of genes significantly were up- or downregulated in iFLs, compared with parental control. By analysis of the threefold changed genes of the microarray data, iFLs and normal fat cells showed the general degree of gene expression overlap. Using the Ontology database for enrichment analysis, we found that the key signaling pathways involved in fat differentiation were significantly changed in iFLs, compared with those in parental MDA-MB-468 cells. Moreover, we examined mRNA levels of a panel of factors reported to be involved in fat differentiation. We found that, in iFLs induced by the reprogramming treatment of ROCK–mTOR inhibitors, expressions of a set of fat differentiation factors were increased by greater than tenfold (Fig. 3c), suggesting that these factors may be involved in breast cancer fat cell conversion.
a Heat map of genes differentially expressed in global RNA-microarray analysis was performed on MDA-MB-468, iFLs, and positive control adipocytes. b Hierarchical clustering was performed. Statistical analysis was performed from three independent experiments. A significant difference between iFLs and their parental cells (p < 0.001), and no difference between iFLs and the control adipocytes (p > 0.5). c qRT-PCR results show relative mRNA levels of defined fat differentiation factors in control and iFLs (n = 3 experiments)
ROCK1/2 and mTORC1 shRNAs generate induced adipocytes
Both ROCK and mTOR are serine/threonine protein kinases. ROCK is a kinase belonging to the AGC (PKA/PKG/PKC) family, including ROCK1 and ROCK2. mTOR interacts with other proteins and serves as a core component of two protein complexes, mTOR complex 1 and mTOR complex 2. To determine if the ROCK and mTOR pathways are involved in breast cancer conversion, we examined the effects of ROCK and mTOR knockdown on breast cancer conversion (Fig. 4a). Lentiviral constructs expressing short hairpin RNA against ROCK1/2 and mTORC1 (Raptor)/C2 (Rictor) were transfected into MDA-MB-468 cells. We found that knockdown of ROCK1, ROCK2, or mTORC1 (Raptor) could reprogram breast cancer cells into iFLs, while mTORC2 did not (Fig. 4b). iFLs showed with mature adipocyte morphology that displayed Oil-Red-O positive staining. Combination treatment of ROCK1/2 and mTORC1 shRNAs converted breast cancer cells to iFLs with kinetics and conversion efficiency similar to ROCK–mTOR inhibitors (Fig. 4c). Thus, we conclude that the ROCK1/2 and mTORC1 kinases are involved in conversion of breast cancer cells.
a Western blot analysis from MDA-MB-468 cells showed that shRNA1 and shRNA2 of ROCK1/2 and mTORC1 (Raptor)/C2 (Rictor) are sufficient to inhibit ROCK1/2, Raptor, or Rictor expression, respectively. Expressions of downstream targets pS6 for Raptor and pAKT1-S473 for Rictor were reduced by Raptor or Rictor shRNAs, respectively. R1: ROCK1, R2: ROCK2. b MDA-MB-468 cells were transduced with ROCK1/2 and mTOR1/2 shRNAs and then cultured in induction medium. Quantitation of induced cells was counted by Oil-Red-O positive staining. The histogram represents the ratio of normalized data between the treatment with shRNAs or DMSO. R1: ROCK1, R2: ROCK2, m1: mTORC1. c Kinetic analysis of iFLs from MDA-MB-468 cells after induction with ROCK1/2-mTORC1 shRNAs in induction medium. Quantitation of induced cells was counted by Oil-Red-O positive staining. Quantitative data are the mean ± SEM from three independent experiments. d Lentiviral shRNAs of AKT1 and IRS1 were expressed in MDA-MB-468 cells. e MDA-MB-468 cells were transduced with AKT1 and IRS1 shRNAs and then cultured in induction medium. Positive iPLs were counted by RFP-positive staining on day 7, and iFLs by Oil-Red-O positive staining on week 4. Quantitative data are the mean ± SEM from three independent experiments
Rapamycin, via inhibition of mTORC1, will also activate a negative feedback loop involving IRS1 driving AKT activation34,35,36,37. To investigate the role of this feedback loop, we expressed lentiviral shRNAs of AKT1 and IRS1 in MDA-MB-468 cells (Fig. 4d). We found that rapamycin-induced reprogramming was reduced by knockdown of AKT1 or IRS1 (Fig. 4e), while knockdown of AKT1 or IRS1 only did not reprogram breast cancer cells into iFLs. These results indicated that inhibition of AKT or IRS1 plays a role in rapamycin-induced reprogramming.
Loss of proliferation and tumorigenicity in induced adipocytes
Next, we examined iFL proliferation. Trypan blue exclusion assays were performed after treating the cells with ROCK–mTOR inhibitors. We observed that ROCK–mTOR inhibitor treatment reduced cell proliferation in a time-dependent manner in breast cancer cells, but not iFLs (Fig. 5a, b). We further evaluated clonogenic formation in the soft agar assay. The mature phenotype of iFLs was accompanied by cessation of proliferation and loss of clonogenic formation ability (Fig. 5c, d). Interestingly, iFLs did not regain proliferation ability or showed signs of further growth following 4 weeks in replacement of induction medium with maintenance medium.
a, b Breast cancer 4T1 cells and iFLs (3 weeks induction) were treated with R + Y. Proliferating cells (viable) were evaluated by the trypan blue dye exclusion assay at day 4 after treatment. Shown are the mean fold change in cell number relative to the 24 h time point and SEM from three treatments. a Cell proliferation change at day 4; b Time course. c, d iFLs (3 weeks induction) from 4T1 were seeded for the soft agar assay. Quantitative data are the mean ± SEM from three independent experiments
To determine tumorigenic properties in vivo, parental MDA-MB-468 cells, iPLs and iFLs (1.5 × 106) were implanted into SCID mice, and tumor formation was monitored for 12 weeks (Fig. 6a, b). No tumors formed following iFL transplantation up to 12 weeks (Fig. 6a). Transplantation of breast cancer cells treated by induction medium without ROCK–mTOR inhibitors resulted in tumor formation in ten of ten mice after 2–3 weeks. Reprogramming MDA-MB-468 cells to iPLs suppressed tumor development and five of ten mice generated tumors, suggesting that induced progenitors are still able to form tumors and require further treatment. Tumors from parental breast cancer cells and iPLs showed the similar morphological features by pathological analysis (Fig. 6c). In addition, there were no significant changes in tumor volume. Thus, we conclude that reprograming breast cancer cells to adipocytes can abrogate the parental tumor cell tumorigenicity.
a, b Parental MDA-MB-468 (Control), iPLs, and iFLs were implanted into mice and the tumor formation was measured. c Haematoxylin and eosin stain for MDA-MB-468 and iPL graft tumors in mice. The xenograft tumors were stained as homogeneous cell mass. The tumor phenotypes did not show difference in MDA-MB-468 and iPL xenograft tumors. d, e Breast cancers generated by 4T1 cells in mice were surgically removed when the primary tumors had reached ~500 mm3. Greater than 95% of each tumor was resected and a small fragment of tumor, the largest being 2 mm3, was not removed to model postoperative residual breast cancers. ROCK–mTOR inhibitors (R + Y) with adipocyte induction medium in the MDD pellet were administered directly into the resection cavity. R + Y without induction medium, medium only in the MDD pellets or no treatment was used as controls. Tumor recurrence (d) and mouse survival (e) were measured (n = 10). f Postoperative residual GFP-4T1 tumors were treated by ROCK–mTOR inhibitors (R + Y) with adipocyte induction medium. GFP-positive cells expressed the adipocyte markers perilipin 3 weeks after transplantation (300 cells counted per section, n = 6). The scale bar represents 10 μm. g Ki67 (IHC)-positive cells were evaluated in postoperative breast tissues treated with R + Y and induction medium. Normal breast tissue was as a control. Ki67-positive cells from recurred tumors treated by R + Y without induction medium, medium only, or no treatment were also examined
Reprogramming orthotopic breast cancer prevents local recurrence
Despite advances in varieties of treatment modalities for breast cancer, local relapse remains a clinically significant problem, especially in patients who underwent breast-conserving therapy. Considering that surgery treatment is essential in breast cancer therapy, we examined whether a direct targeting of the tumor by reprogramming therapy in vivo could prevent cancer local recurrence. We used a highly reproducible mouse model of local breast cancer recurrence following surgical resection of orthotopic 4T1 breast tumor xenografts38,39. 4T1 cells generated tumors in SCID mice were surgically removed when the primary tumors had reached 500 mm3. Greater than 95% of each tumor was resected and a small fragment of tumor, the largest being 2 mm3, was not removed to model residual tumor cells left after breast-conserving surgery40,41,42. Matrix-driven delivery (MDD) pellet of ROCK–mTOR inhibitors with induction medium was prepared by Innovative Research of America. The finished pellet with a biodegradable matrix effectively and continuously releases the active product in the animal for 3 weeks. We implanted the mTOR–ROCK inhibitors with or without adipocyte induction medium in the MDD pellet into the resection cavity of xenografts. Controls included no treatment, induction medium only, and ROCK–mTOR inhibitors without induction medium. Tumor recurrence and mouse survival were measured (Fig. 6d, e, n = 10). The results showed that local recurrence was observed within 2–3 weeks in ten of ten mice without treatment or with induction medium only (Fig. 6d). No tumor recurrence was observed in tumors treated with mTOR–ROCK inhibitor and induction medium treatment up to 12 weeks (0/10 mice). Pathological analysis showed that there were no tumor cells detected in this treatment group. Immunohistochemistry (IHC) analysis revealed that 90% of GFP-positive cells expressed an adipocyte marker perilipin 3 weeks after ROCK–mTOR with induction medium treatment (Fig. 6f), suggesting the GFP cancer cells can be converted into adipocytes in vivo. Flow cytometry analysis revealed that ~60% of the implanted GFP-positive cells survived under ROCK–mTOR inhibitors with induction medium treatment for 3 weeks. We calculated the survived cell yield as the percentage of GFP-positive cells in relation to the initial number of breast cancer cells. This treatment did not affect cell proliferation compared with normal breast tissue control (Fig. 6g). In mTOR–ROCK inhibitors without induction medium treatment, 7/10 mice displayed tumor recurrence, indicating that there is only the limited effect on breast cancer treatment when using mTOR–ROCK inhibitors alone (direct chemotherapy). Recurred tumors from ROCK–mTOR without induction medium, medium only, or no treatment were examined and GFP-positive cells did not express perilipin. Dissected tumors revealed a reduction in Ki67-positive cells in tumors from ROCK–mTOR inhibitors without induction medium treatment compared with those from medium only or no treatment control (Fig. 6g). Taken together, these results indicate that the reprogramming therapy of mTOR–ROCK inhibitors prevents breast cancer local recurrence.
Discussion
We screened a kinase inhibitor library and found that a combination of the ROCK–mTOR kinase inhibitors can substitute for all transcription factors to reprogram breast cancer cells. This conversion is highly efficient and fast, yielding progenitors and more mature adipocytes. Induced adipocytes lose proliferation and tumorigenicity. Furthermore, reprogramming treatment prevents breast cancer local recurrence in mice. Reprogramming breast cancers by kinase inhibitors are a transgene-free method and ROCK–mTOR inhibitors have been used in patients. Therefore, this finding may have wide-spread impact in our understanding and development of breast cancer therapy.
ROCK1 is mainly expressed in the lung, liver, spleen, kidney, and testis, while ROCK2 is distributed mostly in the brain and heart. By acting on the cytoskeleton, ROCK is involved in regulating the shape and movement of cells and also regulates cellular immortalization and differentiation43,44,45,46. A recent report shows that ROCK1 inhibition promotes the self-renewal of cancer stem cells47. mTOR regulates cell growth, proliferation, protein synthesis, and transcription48,49,50,51,52,53,54. ROCK inhibitors are already used as antitumor drugs in patients, while mTOR inhibitors are used to prevent transplant rejection, promote longevity, and treat cancer in clinical trials. Thus, reprogramming strategy of ROCK–mTOR inhibitors is expected to have a low risk in vivo and a new, inhibitor-based, reprogramming therapy could easily and rapidly move toward clinical trials. We found that reprogramming breast cancer cells to progenitors, which decreases tumor development but still generates tumors (five of ten mice). Therefore, induced progenitors require further treatment. In the future studies, we will examine effects of radiation and chemotherapy on the induced progenitors.
One key issue of reprogramming therapy is that reprogrammed cells may be reverted back to cancer cells and form a tumor in vivo. We found that induced adipocytes do not show signs of further proliferation following 4 weeks in replacement of induction medium with maintenance medium. Although we did not observe that the reprogrammed cells revert back, we could not rule out reprogrammed cells reverting and forming a tumor. Additional experiments with different genetic background samples and long-term in vivo observation need to address this issue.
Delineating the molecular mechanism behind breast cancer conversion will greatly aid further development of the method. Gene expression results suggest that ROCK–mTOR may act as a “master regulator” to coordinate a set of defined factors in blocking cell reprogramming in physiological conditions. Consistent with this view, ROCK–mTOR kinases regulate both iPSC reprogramming and cellular differentiation, implicating a general mechanism of reprogramming, where loss of ROCK–mTOR may generate lineage progenitors that develop complete and functional lineages under a variety of induction medium. In previous studies, we found that cellular senescence is a key step in the conversion of human fibroblasts into functional neural cells, including three neural lineages (astrocytes, neurons, and oligodendrocytes)23,24. By genetically manipulating the senescence process, we provided a new method for converting most human fibroblasts into neural cells in only 1–2 weeks. Both ROCK and mTOR kinases are involved in senescence and proliferation processes, implicating that the reprogramming process may also be regulated through the kinase–senescence signaling pathways.
The reprogramming-based therapy will provide new avenues for development of therapeutics for breast cancer. ROCK–mTOR inhibitors are already used as antitumor drugs in patients and promise few or no side effects in reprogramming. We expect the adipocytes generated by the ROCK–mTOR inhibitors are “safe” in vivo. The results show that the reprogramming treatment of kinase inhibitors does not affect normal adipocytes, supporting the safety of the reprogramming treatment. Therefore, this strategy is expected to develop a novel, safe, and effective kinase inhibitor-based reprogramming method to combine with conventional therapy for clinical use.
Methods
Cell culture
Human breast cancer cells, MDA-MB-468, MDA-MB-231, and HCC2157 (ATCC), were routinely cultured in 60 mm dish (MidSci) in Dulbecco’s modified Eagle medium (Hyclone) containing 10% fetal bovine serum (Millipore-Sigma), non-essential amino acids (Invitrogen), and penicillin/streptomycin (Invitrogen) at 37 °C in 5% CO2. Human adipocytes from ATCC were routinely cultured in adipocyte medium.
Breast cancer cell reprogramming
Breast cancer cells were plated at a density of 3.0 × 104 cells cm−2 on microscope glass coverslips coated with matrigel (BD) in 35 mm dishes. For kinase inhibitor screening experiments, we used 2 µM protein kinase inhibitor from a library (Calbiochem, 355 inhibitors). The media were changed to defined induction medium including DMEM/F12 (2% FBS) plus 1 µM dexamethasone, 0.5 μM isobutylmethylxanthine, and 200 µM indomethacin (Millipore-Sigma). For adipocyte induction, we used adipocyte induction medium including conditional adipocyte medium (Zen-Bio, Inc) plus 2 μM Y27632 and Rapamycin, 1 μM dexamethasone, 0.5 μM isobutylmethylxanthine, and 200 μM indomethacin. Cells were further grown under such conditions for up to 21 days. Fat differentiation was visualized with Oil-Red-O (Millipore-Sigma) stain as previously described55,56.
qRT-PCR
Total RNA was extracted using Trizol isolation system (Invitrogen) according to the manufacturer’s instructions. Total RNA was treated with DNase I (Qiagen) to prevent DNA contamination. Two micrograms of RNA were reverse transcribed using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche). Reverse transcription and real-time PCR were performed as a standard procedure.
Microarray analysis
Microarray analysis was performed at the Washington University Genome Center. Briefly, Illumina HumanHT-12 v4 Expression BeadChip was used and samples were labeled by biotin. The direct hybridization assay was performed and the data were scanned on the BeadArray Reader. Scanned images were quantitated by Illumina Beadscan, v3. Quantitative data were imported into Illumina GenomeStudio software and normalized by Illumina’s quantile method. The quantile-normalized background-subtracted data were calculated in excel. The data were filtered based on the average signal, and set the baseline as 50 so that only the genes of which average signal >50 could be used for further analysis. To each gene, maximum and minimum signal values were selected, and then divided between them. Only the genes of the division value >3 were selected and considered as differently expressed. All of the differently expressed genes were clustered by using MeV software.
To determine the relationship among breast cancer cells and iFLs, each sample was analyzed respectively. Total RNA from cultured subcutaneous Adipocytes (Zen-Bio) is as a positive control. Data were filtered based on average signal and baseline was set as 10 to increase the sensitivity. Differently expressed genes were selected by a threefold change as the threshold. To examine the potential adipocyte differentiation pathways relevant to the immortalization, all fat differentiation genes from Gene Ontology Website (http://www.geneontology.org/, GO: 0030182) were compared with the microarray data. For gene enrichment analysis, we used web-based Gorilla program (http://cbl-gorilla.cs.technion.ac.il/). Thus, these results will greatly help for understanding mechanisms of breast cancer cell reprogramming.
Trypan blue dye exclusion and soft agar assays
Parental breast cancer cells and iFLs were seeded at a density of 10,000 cells/well in 12 well. Cells were counted with a ViCell cell viability analyzer (Beckman Coulter). Cell proliferation was normalized to the percentage of control. Three independent experiments with triplicates were performed for each cell line. For the soft agar assay, cells (10,000/dish) were suspended in 2 ml of 0.3% agar and poured over a 3 ml 0.6% solidified layer of agar in 60 mm dishes. Cells were incubated for about 14–16 days until colonies were large enough to visualize. Colonies were observed under phase contrast microscope. All data were normalized relative to the control. Experiments were performed in triplicate.
Breast cancer, iPL, and iFL cell transplantation and cancer recurrence model in mice
Animal experiments were performed according to institutional guidelines for animal welfare. Female NOD.SCID/NCR mice of 6–8 weeks of age were purchased from NCI-Frederick Animal Production Program (Frederick, MA, USA). In all, 2 × 106 breast cancer cells, iPLs and iFLs in 0.1 ml PBS, were mixed with equal volume of matrigel. The cell mixture was implanted into mouse breast fat pad. The tumors became rigid and the volume of tumor ((L × W2)/2) was measured. At the end of treatment, the mice were killed and the tumors were excised and processed to paraffin section.
For breast cancer local recurrence experiments, we used a mouse model of local recurrence following surgical resection of orthotopic tumors. Breast cancer generated by MDA-MB-468 cells in mice were surgically removed when the primary tumors had reached ~500 mm3. Greater than 95% of each tumor was excised and a small fragment of tumor, the largest being 2 mm3, was not removed to model postoperative residual breast cancers. Matrix-driven delivery (MDD) pellet of ROCK–mTOR inhibitors with adipocyte induction medium was prepared by Innovative Research of America. We implanted the MDD pellet including mTOR–ROCK inhibitors (2 µM R + Y per day for 21 days) with or without induction medium into the resection cavity of breast cancers. Adipocyte induction medium includes 1 µM dexamethasone, 0.5 mM isobutylmethylxanthine, and 200 µM indomethacin with conditional adipocyte medium (Zen-Bio, Inc). MDD pellet with induction medium, inhibitor only, and no treatment are as a control. Mouse breast tissue and tumors were excised and sectioned on a cryostat for IHC assay analyses57,58,59.
Change history
07 October 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41419-022-05311-9
References
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Papp, B. & Plath, K. Reprogramming to pluripotency: stepwise resetting of the epigenetic landscape. Cell Res. 21, 486–501 (2011).
Cherry, A. B. & Daley, G. Q. Reprogramming cellular identity for regenerative medicine. Cell 148, 1110–1122 (2012).
Izgi, K., Canatan, H. & Iskender, B. Current status in cancer cell reprogramming and its clinical implications. J. Cancer Res. Clin. Oncol. 143, 371–383 (2017).
Powers, S. & Pollack, R. E. Inducing stable reversion to achieve cancer control. Nat. Rev. Cancer 16, 266–270 (2016).
Lang, J. Y., Shi, Y. & Chin, Y. E. Reprogramming cancer cells: back to the future. Oncogene 32, 2247–2248 (2013).
Hochedlinger, K. et al. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 18, 1875–1885 (2004).
Ramos-Mejia, V., Fraga, M. F. & Menendez, P. iPSCs from cancer cells: challenges and opportunities. Trends Mol. Med. 18, 245–247 (2012).
Zhang, X., Cruz, F. D., Terry, M., Remotti, F. & Matushansky, I. Terminal differentiation and loss of tumorigenicity of human cancers via pluripotency-based reprogramming. Oncogene 32, 2249–2260 (2013). 2260.
Carette, J. E. et al. Generation of iPSCs from cultured human malignant cells. Blood 115, 4039–4042 (2010).
Utikal, J., Maherali, N., Kulalert, W. & Hochedlinger, K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J. Cell Sci. 122, 3502–3510 (2009).
Miyoshi, N. et al. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc. Natl Acad. Sci. USA 107, 40–45 (2010).
Chang, G. et al. Linking incomplete reprogramming to the improved pluripotency of murine embryonal carcinoma cell-derived pluripotent stem cells. PLoS ONE 5, e10320 (2010).
Choi, S. M. et al. Reprogramming of EBV-immortalized B-lymphocyte cell lines into induced pluripotent stem cells. Blood 118, 1801–1805 (2011).
Hu, K. et al. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood 117, e109–e119 (2011).
Lin, S. L. et al. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA 14, 2115–2124 (2008).
Amson, R. et al. Reciprocal repression between P53 and TCTP. Nat. Med. 18, 91–99 (2011).
Amson, R., Karp, J. E. & Telerman, A. Lessons from tumor reversion for cancer treatment. Curr. Opin. Oncol. 25, 59–65 (2013).
Gascard, P. et al. Epigenetic and transcriptional determinants of the human breast. Nat. Commun. 6, 6351 (2015).
Ho, R., Chronis, C. & Plath, K. Mechanistic insights into reprogramming to induced pluripotency. J. Cell. Physiol. 226, 868–878 (2011).
Han, S. S., Williams, L. A. & Eggan, K. C. Constructing and deconstructing stem cell models of neurological disease. Neuron 70, 626–644 (2011).
Robinton, D. A. & Daley, G. Q. The promise of induced pluripotent stem cells in research and therapy. Nature 481, 295–305 (2012).
Sun, C. K. et al. Senescence impairs direct conversion of human somatic cells to neurons. Nat. Commun. 5, 4112 (2014).
Zhou, D. et al. Conversion of fibroblasts to neural cells by p53 depletion. Cell Rep. 9, 2034–2042 (2014).
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007).
Yang, Q. Cellular senescence, telomere recombination and maintenance. Cytogenet. Genome Res. 122, 211–218 (2008).
Yang, Q. et al. Functional diversity of human protection of telomeres 1 isoforms in telomere protection and cellular senescence. Cancer Res. 67, 11677–11686 (2007).
Beausejour, C. M. et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 22, 4212–4222 (2003).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Utikal, J. et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145–1148 (2009).
Li, H. et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460, 1136–1139 (2009).
Lai, R. C., Choo, A. & Lim, S. K. Derivation and characterization of human ESC-derived mesenchymal stem cells. Methods Mol. Biol. 698, 141–150 (2011).
Feng, J., Mantesso, A., De, B. C., Nishiyama, A. & Sharpe, P. T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl Acad. Sci. USA 108, 6503–6508 (2011).
Copps, K. D., Hancer, N. J., Qiu, W. & White, M. F. Serine 302 phosphorylation of mouse insulin receptor substrate 1 (IRS1) is dispensable for normal insulin signaling and feedback regulation by hepatic S6 kinase. J. Biol. Chem. 291, 8602–8617 (2016).
Gao, S., Duan, C., Gao, G., Wang, X. & Yang, H. Alpha-synuclein overexpression negatively regulates insulin receptor substrate 1 by activating mTORC1/S6K1 signaling. Int. J. Biochem. Cell Biol. 64, 25–33 (2015).
Duan, L. et al. The prolyl peptidases PRCP/PREP regulate IRS-1 stability critical for rapamycin-induced feedback activation of PI3K and AKT. J. Biol. Chem. 289, 21694–21705 (2014).
Leontieva, O. V., Demidenko, Z. N. & Blagosklonny, M. V. Rapamycin reverses insulin resistance (IR) in high-glucose medium without causing IR in normoglycemic medium. Cell Death Dis. 5, e1214 (2014).
Danna, E. A. et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 64, 2205–2211 (2004).
Pulaski, B. A. & Ostrand-Rosenberg, S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 58, 1486–1493 (1998).
Smith, M. J. et al. Mechanisms driving local breast cancer recurrence in a model of breast-conserving surgery. Ann. Surg. Oncol. 15, 2954–2964 (2008).
Coffey, J. C. et al. Phosphoinositide 3-kinase accelerates postoperative tumor growth by inhibiting apoptosis and enhancing resistance to chemotherapy-induced apoptosis. Novel role for an old enemy. J. Biol. Chem. 280, 20968–20977 (2005).
Lei, N. et al. Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model. Nanoscale 4, 5686–5693 (2012).
Wong, C. C., Wong, C. M., Au, S. L. & Ng, I. O. RhoGTPases and Rho-effectors in hepatocellular carcinoma metastasis: ROCK N’Rho move it. Liver Int. 30, 642–656 (2010).
Micuda, S., Rosel, D., Ryska, A. & Brabek, J. ROCK inhibitors as emerging therapeutic candidates for sarcomas. Curr. Cancer Drug Targets 10, 127–134 (2010).
Narumiya, S., Tanji, M. & Ishizaki, T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastas. Rev. 28, 65–76 (2009).
Olson, M. F. Applications for ROCK kinase inhibition. Curr. Opin. Cell Biol. 20, 242–248 (2008).
Castro, D. J., Maurer, J., Hebbard, L. & Oshima, R. G. ROCK1 inhibition promotes the self-renewal of a novel mouse mammary cancer stem cell. Stem Cells 31, 12–22 (2013).
Chen, T. et al. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell 10, 908–911 (2011).
Menendez, J. A., Vellon, L., Oliveras-Ferraros, C., Cufi, S. & Vazquez-Martin, A. mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging. Cell Cycle 10, 3658–3677 (2011).
Cornu, M., Albert, V. & Hall, M. N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 23, 53–62 (2013).
Kaeberlein, M. Lessons on longevity from budding yeast. Nature 464, 513–519 (2010).
Weichhart, T. Mammalian target of rapamycin: a signaling kinase for every aspect of cellular life. Methods Mol. Biol. 821, 1–14 (2012).
McCormick, M. A., Tsai, S. Y. & Kennedy, B. K. TOR and ageing: a complex pathway for a complex process. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 17–27 (2011).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Matushansky, I. et al. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J. Clin. Invest. 117, 3248–3257 (2007).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Sun, C. K. et al. Phosphorylation of ribosomal protein S6 confers PARP inhibitor resistance in BRCA1-deficient cancers. Oncotarget 5, 3375–3385 (2014)
Xiang, T. et al. Targeting the Akt/mTOR pathway in Brca1-deficient cancers. Oncogene 30, 2443–2450 (2011).
Zeng, S. et al. Telomere recombination requires the MUS81 endonuclease. Nat. Cell Biol. 11, 616–623 (2009).
Acknowledgements
We thank Buck Rogers and Xiaowei Wang for proofreading this manuscript. This work is supported in part by grants from Children’s Discovery Institute (MC-II-2016-511, Q.Y.), Institute of Clinical and Translational Sciences (1UL1TR00234501, Q.Y.), and Medical Scientific Research Foundation of Guangdong Province in China (A2017558, J.Y.).
Author information
Authors and Affiliations
Contributions
Q.Y. generated the hypotheses, designed the experiments, and analyzed the data. J.Y., F.Z. and M.Y. performed the experiments and generated the data. Q.Y. wrote the manuscript.
Corresponding author
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by J. Chipuk
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41419-022-05311-9
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, J., Zhang, F., You, M. et al. RETRACTED ARTICLE: Identification of protein kinase inhibitors to reprogram breast cancer cells. Cell Death Dis 9, 915 (2018). https://doi.org/10.1038/s41419-018-1002-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-018-1002-2
This article is cited by
-
Tumor reversion: a dream or a reality
Biomarker Research (2021)
-
Retraction Note: Reprogramming glioblastoma multiforme cells into neurons by protein kinase inhibitors
Journal of Experimental & Clinical Cancer Research (2021)