Abstract
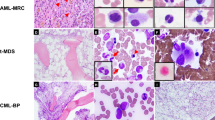
Recurrent fusion genes (FGs) with clinical significances in leukemias are mainly mutually exclusive, and the coexistence of different FGs has been rarely reported. In this study, we retrospectively analyzed the incidence, genetic characteristics, and prognosis of leukemias with concurrent pathogenic FGs, which commonly reported in hematological malignancies in 8226 leukemia patients. A total of 25 patients with coexistence of double FGs were identified, accounting for 0.30% of all cases enrolled. More than half of the cases (14/25, 56%) were diagnosed as chronic myeloid leukemia in accelerated or blast phase, another six and five cases were acute myeloid leukemia and acute lymphocytic leukemia, respectively. Most cases (20/25, 80%) carried constitutively activated tyrosine kinases FGs (BCR-ABL1 or ETV6-PDGFRB) and transcription factors associated FGs simultaneously. Of the 11 patients with contemporaneous karyotype, 5 (45%) showed visible chromosomal abnormalities corresponding to both FGs. The concurrency of FGs was often associated with disease progressions. The prognosis was pessimistic for patients with concurrent FGs, even with the combination of targeted therapy and chemotherapy. Performing allogeneic hematopoietic stem cell transplantation as soon as possible after complete remission can ameliorate the dismal prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. TheWorld Health Organization classification of neoplastic diseases of the haematopoietic and lymphoid tissues: report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Histopathology. 2000;36:69-86.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114:937–51.
Chen X, Wang F, Zhang Y, Wang M, Tian W, Teng W, et al. Panoramic view of common fusion genes in a large cohort of Chinese de novo acute myeloid leukemia patients. Leuk Lymphoma 2019;60:1071–8.
Chen X, Wang F, Zhang Y, Wang M, Tian W, Teng W, et al. Retrospective analysis of 36 fusion genes in 2479 Chinese patients of de novo acute lymphoblastic leukemia. Leuk Res 2018;72:99–104.
Zhang Y, Wang F, Chen X, Zhang Y, Wang M, Liu H, et al. CSF3R Mutations are frequently associated with abnormalities of RUNX1, CBFB, CEBPA, and NPM1 genes in acute myeloid leukemia. Cancer 2018;124:3329–38.
Tallman MS, Wang ES, Altman JK, Appelbaum FR, Bhatt VR, Bixby D, et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:721–49.
Radich JP, Deininger M, Abboud CN, Altman JK, Berman E, Bhatia R, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:1108–35.
Alvarnas JC, Brown PA, Aoun P, Ballen KK, Barta SK, Borate U, et al. Acute Lymphoblastic Leukemia, Version 2.2015. J Natl Compr Canc Netw 2015;13:1240–79.
Wang YY, Ding WJ, Jiang F, Chen ZX, Cen JN, Qi XF, et al. Coexistence ofp210(BCR-ABL) and CBFbeta-MYH11 fusion genes in myeloid leukemia: a report of 4 cases. Oncol Lett 2017;14:5171–8.
Balatzenko G, Guenova M, Kalinova I, Belcheva M, Hristozova H, Kaleva V. Simultaneous occurrence of ETV6-RUNX1 and BCR-ABL1 (e1a2) transcripts in a child with B-cell acute lymphoblastic leukemia. Cancer Genet 2013;206:97–101.
Zhang S, Zhou W, Li Y, Yu S, Xue M, Qiao Y, et al. Co-expression of AML1-ETO and PML-RARa following treatment of de novo acute myeloid leukemia with AML1-ETO. Leuk Lymphoma 2019;60:1316–9.
Dai HP, Xue YQ, Wu LL, Pan JL, Gong YL, Wu YF, et al. p210 BCR/ABL1 as a secondary change in a patient with acute myelomonocytic leukemia (M4Eo) with inv(16). Int J Hematol 2012;96:814–7.
Han E, Lee H, Kim M, Kim Y, Han K, Lee SE, et al. Characteristics of hematologic malignancies with coexisting t(9;22) and inv(16) chromosomal abnormalities. Blood Res 2014;49:22–8.
Salem A, Loghavi S, Tang G, Huh YO, Jabbour EJ, Kantarjian H, et al. Myeloid neoplasms with concurrent BCR-ABL1 and CBFB rearrangements: A series of 10 cases of a clinically aggressive neoplasm. Am J Hematol 2017;92:520–8.
Speck NA, Gilliland DG. Core-binding factors in haematopoiesis and leukaemia. Nat Rev Cancer. 2002;2:502–13.
Yin CC, Cortes J, Barkoh B, Hayes K, Kantarjian H, Jones D. t(3;21)(q26; q22) in myeloid leukemia: an aggressive syndrome of blast transformation associated with hydroxyurea or antimetabolite therapy. Cancer 2006;106:1730–8.
Scandura JM, Boccuni P, Cammenga J, Nimer SD. Transcription factor fusions in acute leukemia: variations on a theme. Oncogene 2002;21:3422–44.
Van Vlierberghe P, van Grotel M, Tchinda J, Lee C, Beverloo HB, van der Spek PJ, et al. The recurrent SET-NUP214 fusion as a new HOXA activation mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood 2008;111:4668–80.
Sotoca AM, Prange KH, Reijnders B, Mandoli A, Nguyen LN, Stunnenberg HG, et al. The oncofusion protein FUS-ERG targets key hematopoietic regulators and modulates the all-trans retinoic acid signaling pathway in t(16;21) acute myeloid leukemia. Oncogene 2016;35:1965–76.
O’Neil J, Shank J, Cusson N, Murre C, Kelliher M. TAL1/SCL induces leukemia by inhibiting the transcriptional activity of E47/HEB. Cancer Cell 2004;5:587–96.
Slape C, Chung YJ, Soloway PD, Tessarollo L, Aplan PD. Mouse embryonic stem cells that express a NUP98-HOXD13 fusion protein are impaired in their ability to differentiate and can be complemented by BCR-ABL. Leukemia 2007;21:1239–48.
Meyer C, Burmeister T, Groger D, Tsaur G, Fechina L, Renneville A, et al. The MLL recombinome of acute leukemias in 2017. Leukemia 2018;32:273–84.
Manara E, Baron E, Tregnago C, Aveic S, Bisio V, Bresolin S, et al. MLL-AF6 fusion oncogene sequesters AF6 into the nucleus to trigger RAS activation in myeloid leukemia. Blood 2014;124:263–72.
Aplan PD, Lombardi DP, Reaman GH, Sather HN, Hammond GD, Kirsch IR. Involvement of the putative hematopoietic transcription factor SCL in T-cell acute lymphoblastic leukemia. Blood 1992;79:1327–33.
Chen B, Jiang L, Zhong ML, Li JF, Li BS, Peng LJ, et al. Identification of fusion genes and characterization of transcriptome features in T-cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA 2018;115:373–8.
Liu Y, Easton J, Shao Y, Maciaszek J, Wang Z, Wilkinson MR, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49:1211–8.
Acknowledgements
We thank Lin Zhou, Na Wang, Lan Wang, Xinna Liu, and Qing Yin from Divison of Pathology & Laboratory Medicine of Hebei Yanda Lu Daopei Hospital for technical support. We thank Yanli Zhao, Yue Lu, Xingyu Cao, Jiarui Zhou, Ruijuan Sun, Jianping Zhang, Min Xiong, and Zhijie Wei from Bone Marrow Transplantation Department and Xian Zhang, Junfang Yang, Fanyong Lv from Hematology Department of Hebei Yanda Lu Daopei Hospital for patient and sample supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Wang, F., Wang, T. et al. The incidence, genetic characteristics, and prognosis of leukemia with concurrent pathogenic fusion genes: a series of 25 cases from a large cohort of leukemia patients. Cancer Gene Ther 27, 89–97 (2020). https://doi.org/10.1038/s41417-019-0147-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-019-0147-1
This article is cited by
-
Identification of novel PIEZO1::CBFA2T3 and INO80C::SETBP1 fusion genes in an acute myeloid leukemia patient by RNA-seq
Molecular Biology Reports (2023)
-
Genomic and clinical findings in myeloid neoplasms with PDGFRB rearrangement
Annals of Hematology (2022)
-
Fusion gene map of acute leukemia revealed by transcriptome sequencing of a consecutive cohort of 1000 cases in a single center
Blood Cancer Journal (2021)