Abstract
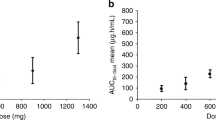
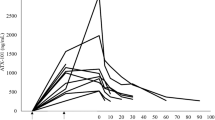
We conducted a phase 1 trial for single-dose intravenous Ad5CRT, a replication-defective adenovirus vector expressing HSVtk (herpes simplex virus thymidine kinase) modulated by a specific trans-splicing ribozyme that targets human telomerase reverse transcriptase (hTERT)-encoding RNAs. Dose-limiting toxicities (DLTs) were evaluated in 15 patients at dose levels of 0.1–2 × 1012 virus particles. Patients well tolerated study treatment. During the DLT evaluation period, none of the 15 patients developed any grade 4 toxicities or treatment discontinuation that was related to agents investigated by this trial. The most frequent treatment-related adverse event was fever/chill (26.7%). Of the 18 patients, no patients achieved a partial or complete response, and the median progression-free survival for 18 patients was 1.1 months (95% CI, 1.0–1.3) and the results suggest no clinical benefit from this treatment. Ad5CRT’s circulating virus half-life was approximately 10 min. Maximum tolerated dose was 2 × 1012 virus particles. Single-dose intravenous Ad5CRT was feasible and well tolerated in patients with gastrointestinal cancer liver metastasis. Ad5CRT did not provide meaningful clinical benefit, and the reason for the lack of efficacy was not entirely clear because no pharmocodynamic assessment was made.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Garcia-Carbonero R, Salazar R, Duran I, Osman-Garcia I, Paz-Ares L, Bozada JM, et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J Immunother Cancer. 2017;5:71–83.
Sun L, Funchain P, Song JM, Rayman P, Tannenbaum C, Ko J, et al. Talimogene Laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III-IV melanoma: a case series. J Immunother Cancer. 2018;6:36–42.
Hong SH, Jeong JS, Lee YJ, Jung HI, Cho KS, Kim CM, et al. In vivo reprogramming of hTERT by trans-splicing ribozyme to target tumor cells. Mol Ther. 2008;16:74–80.
Suzuki K, Kashimura H, Ohkawa J, Itabashi M, Watanabe T, Sawahata T, et al. Expression of human telomerase catalytic subunit gene in cancerous and precancerous gastric conditions. J Gastroenterol Hepatol. 2000;15:744–51.
Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–9.
Sabah M, Cummins R, Leader M, Kay E. Expression of human telomerase reverse transcriptase in gastrointestinal stromal tumors occurs preferentially in malignant neoplasms. Hum Pathol. 2004;35:1231–5.
Dômont J, Pawlik TM, Boige V, Rose M, Weber JC, Hoff PM, et al. Catalytic subunit of human telomerase reverse transcriptase is an independent predictor of survival in patients undergoing curative resection of hepatic colorectal metastases: a multicenter analysis. J Clin Oncol. 2005;23:3086–93.
Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91.
Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–17.
Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–59.
Laurie SA, Bell JC, Atkins HL, Roach J, Bamat MK, O’Neil JD, et al. A phase 1 clinical study of intravenous administration of PV701, an oncolytic virus, using two-step desensitization. Clin Cancer Res. 2016;12:2555–62.
Breitbach CJ, J. Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102.
Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–37.
Funding
This study was supported by National Cancer Center grants (NCC-1610281 and 1810950).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, SJ., Shin, SP., Lee, S.H. et al. Phase I trial of intravenous Ad5CRT in patients with liver metastasis of gastrointestinal cancers. Cancer Gene Ther 26, 174–178 (2019). https://doi.org/10.1038/s41417-018-0055-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0055-9
This article is cited by
-
Synthetic introns enable splicing factor mutation-dependent targeting of cancer cells
Nature Biotechnology (2022)