Abstract
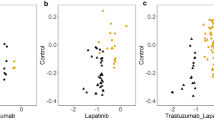
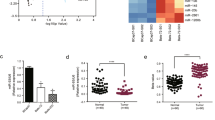
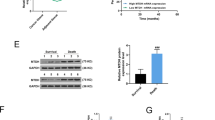
It has been found that microRNAs (miRNAs) play a key role in drug resistance. The purpose of the current study was to investigate the function of miR-182 in trastuzumab resistance in breast cancer cells. The results showed that both breast cancer SKBR3 trastuzumab-resistant cells (SKBR3/TR) and BT474 trastuzumab-resistant cells (BT474/TR) were associated with miR-182 downregulation compared with their parental cells. Ectopic expression of the miR-182 mimic inhibited trastuzumab resistance, decreasing the invasion and migration of these trastuzumab-resistant cells. However, the miR-182 inhibitor increased trastuzumab resistance, cell invasion, and migration in the parental cells. In addition, MET is a directly targeted gene of miR-182 in breast cancer cells. MET knockdown showed an inhibitory effect of trastuzumab resistance on trastuzumab-resistant cells. In contrast, MET overexpression in SKBR3 cells produced an effect that promotes resistance to trastuzumab. Moreover, we revealed that overexpression of miR-182 reduced trastuzumab resistance in trastuzumab-resistant cells due in part to MET/PI3K/AKT/mTOR signaling pathway inactivation. Furthermore, miR-182 could also sensitize SKBR3/TR cells to trastuzumab in vivo. In conclusion, our results suggest that the activation of miR-182 or inactivation of its target gene pathway could be used as a new method to reverse trastuzumab resistance in breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–90.
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, Shi B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929–43.
Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer Basic Clin Res. 2016;10:25–36.
Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer. 2016;8:93–107.
Ieni A, Barresi V, Ricciardi GR, Adamo B, Adamo V, Tuccari G. Prognostic value of androgen receptor expression in triple negative breast carcinomas: personal experience and comments on a review about “Triple-negative breast cancer: treatment challenges and solutions” by Collignon et al. Breast Cancer. 2016;8:157–9.
Dent S, Oyan B, Honig A, Mano M, Howell S. HER2-targeted therapy in breast cancer: a systematic review of neoadjuvant trials. Cancer Treat Rev. 2013;39:622–31.
Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9:16–32.
Maximiano S, Magalhaes P, Guerreiro MP, Morgado M. Trastuzumab in the treatment of breast cancer. BioDrugs Clin Immunother Biopharm Gene Ther. 2016;30:75–86.
Laakmann E, Muller V, Schmidt M, Witzel I. Systemic treatment options for HER2-positive breast cancer patients with brain metastases beyond trastuzumab: a literature review. Breast Care. 2017;12:168–71.
Luque-Cabal M, Garcia-Teijido P, Fernandez-Perez Y, Sanchez-Lorenzo L, Palacio-Vazquez I. Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome it. Clin Med Insights Oncol. 2016;10:21–30.
Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–9.
Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genom Proteom Bioinforma. 2009;7:147–54.
Majumder S, Jacob ST. Emerging role of microRNAs in drug-resistant breast cancer. Gene Expr. 2011;15:141–51.
Ma J, Dong C, Ji C. MicroRNA and drug resistance. Cancer Gene Ther. 2010;17:523–31.
Arya D, Sachithanandan SP, Ross C, Palakodeti D, Li S, Krishna S. MiRNA182 regulates percentage of myeloid and erythroid cells in chronic myeloid leukemia. Cell Death Dis. 2017;8:e2547.
Bian DL, Wang XM, Huang K, Zhai QX, Yu GB, Wu CH. Expression and regulatory effects of microRNA-182 in osteosarcoma cells: a pilot study. Oncol Lett. 2016;11:3040–8.
Liu R, Li J, Teng Z, Zhang Z, Xu Y. Overexpressed microRNA-182 promotes proliferation and invasion in prostate cancer PC-3 cells by down-regulating N-myc downstream regulated gene 1 (NDRG1). PLoS ONE. 2013;8:e68982.
Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27(kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64:3981–6.
Gijsen M, King P, Perera T, Parker PJ, Harris AL, Larijani B, Kong A. HER2 phosphorylation is maintained by a PKB negative feedback loop in response to anti-HER2 herceptin in breast cancer. PLoS Biol. 2010;8:e1000563.
Hu J, Lv G, Zhou S, Zhou Y, Nie B, Duan H, Zhang Y, Yuan X. The downregulation of miR-182 is associated with the growth and invasion of osteosarcoma cells through the regulation of TIAM1 expression. PLoS ONE. 2015;10:e0121175.
Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9:E52.
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers. 2014;6:1769–92.
Rueff J, Rodrigues AS. Cancer drug resistance: a brief overview from a genetic viewpoint. Methods Mol Biol. 2016;1395:1–18.
Kerbel RS. Molecular and physiologic mechanisms of drug resistance in cancer: an overview. Cancer Metastas Rev. 2001;20:1–2.
O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, Duffy MJ, Crown J, O’Donovan N, Slamon DJ. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–502.
Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11:263–75.
Wei Q, Lei R, Hu G. Roles of miR-182 in sensory organ development and cancer. Thorac Cancer. 2015;6:2–9.
Kouri FM, Hurley LA, Daniel WL, Day ES, Hua Y, Hao L, Peng CY, Merkel TJ, Queisser MA, Ritner C, Zhang H, James CD, Sznajder JI, Chin L, Giljohann DA, Kessler JA, Peter ME, Mirkin CA, Stegh AH. miR-182 integrates apoptosis, growth, and differentiation programs in glioblastoma. Genes Dev. 2015;29:732–45.
Chiang CH, Chu PY, Hou MF, Hung WC. miR-182 promotes proliferation and invasion and elevates the HIF-1alpha-VEGF-A axis in breast cancer cells by targeting FBXW7. Am J Cancer Res. 2016;6:1785–98.
Chiang CH, Hou MF, Hung WC. Up-regulation of miR-182 by beta-catenin in breast cancer increases tumorigenicity and invasiveness by targeting the matrix metalloproteinase inhibitor RECK. Biochim Biophys Acta. 2013;1830:3067–76.
McCleese JK, Bear MD, Kulp SK, Mazcko C, Khanna C, London CA. Met interacts with EGFR and Ron in canine osteosarcoma. Vet Comp Oncol. 2013;11:124–39.
Minuti G, Cappuzzo F, Duchnowska R, Jassem J, Fabi A, O’Brien T, Mendoza AD, Landi L, Biernat W, Czartoryska-Arlukowicz B, Jankowski T, Zuziak D, Zok J, Szostakiewicz B, Foszczynska-Kloda M, Tempinska-Szalach A, Rossi E, Varella-Garcia M. Increased MET and HGF gene copy numbers are associated with trastuzumab failure in HER2-positive metastatic breast cancer. Br J Cancer. 2012;107:793–9.
Suzuki Y, Saito Y, Terao M, Terada M, Morioka T, Tsuda B, Okamura T, Niikura N, Tokuda Y. Trastuzumab and chemotherapy after the treatment failure of lapatinib for HER2-positive metastatic breast cancer. Tokai J Exp Clin Med. 2010;35:148–51.
Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3:S7–19.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yue, D., Qin, X. miR-182 regulates trastuzumab resistance by targeting MET in breast cancer cells. Cancer Gene Ther 26, 1–10 (2019). https://doi.org/10.1038/s41417-018-0031-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0031-4
This article is cited by
-
The emerging role of MicroRNA-182 in tumorigenesis; a promising therapeutic target
Cancer Cell International (2023)
-
Landscape of NcRNAs involved in drug resistance of breast cancer
Clinical and Translational Oncology (2023)
-
Coding the noncoding: 2 years of advances in the field of microRNAs and long noncoding RNAs
Cancer Gene Therapy (2021)
-
Breast Cancer Response to Therapy: Can microRNAs Lead the Way?
Journal of Mammary Gland Biology and Neoplasia (2021)
-
PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers
Cell Death & Disease (2020)