Abstract
Programmed death ligand 1 (PD-L1) expression as a predictive biomarker for programmed cell death 1 (PD-1) inhibitor efficacy in gastric cancer (GC) remains controversial. We hypothesised that the conflicting results may be due to the inaccurate assessment of PD-L1 expression using biopsy samples. A total of 191 patients with GC who received radical resection were enrolled. PD-L1 expressions in biopsy and paired resected samples by immunohistochemistry staining were compared according to the number of biopsies. The numbers of PD-L1-positive patients determined by biopsy and resected samples were 89 (46.6%) and 135 (70.1%), respectively. The accordance rate was 64.4% (κ = 0.31). Single biopsy showed a lower accordance rate compared with multiple biopsies. Our study revealed that single biopsy cannot fully reflect PD-L1 expression in the whole tumour in GC. Multiple biopsies are recommended for accurate diagnosis of PD-L1 expression in GC.
Similar content being viewed by others
Background
Immune therapy targeting programmed cell death 1 (PD-1) and its ligand programmed death ligand 1 (PD-L1) demonstrated favourable therapeutic effects in gastric cancer (GC) in several clinical trials.1,2 PD-L1 expression has been considered a potential biomarker for treatment efficacy in several types of cancer, including melanoma and non-small-cell lung carcinoma (NSCLC).3 However, whether PD-L1 expression is a predictive biomarker for PD-1/PD-L1 inhibitor efficacy in GC remains controversial.1,4
GC shows a strong histological heterogeneity in primary lesions, as various histological and differentiation types are frequently observed in the same samples. Intratumoural heterogeneity is often an obstacle for accurate assessment of tumour profiles and determining treatment strategy.5 We hypothesised that PD-L1 expression evaluated by biopsy samples might differ from PD-L1 expression in the whole tumour because of intratumoural heterogeneity. To confirm whether PD-L1 expression evaluated by biopsy specimen accurately reflects its expression in the whole tumour, we compared PD-L1 expression of biopsy samples with those of matched resected samples and examined the correlations between the number of biopsies and accurate diagnosis of PD-L1 expression.
Methods
Patients and samples
A total of 191 patients with advanced GC with tumour depth below the muscularis propria layer were included (Supplemental Table 1). The patients received radical gastrectomy without pretreatment between 2005 and 2014 in Kumamoto University Hospital. Paired biopsy and resected samples from the same patients were used for PD-L1 immunohistochemistry. The study procedures were approved by the institutional review board (No. 1037), which waived the requirement for informed consent because of the retrospective nature of the study.
Immunohistochemistry
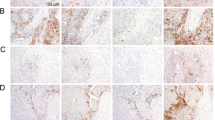
Formalin-fixed paraffin-embedded GC tissue samples were sectioned (5 μm) and deparaffinised. Heat-induced antigen retrieval was performed in antigen retrieval solution (pH 9, Histofine; Nichirei Biosciences, Tokyo, Japan) with a steamer autoclave at 121 °C for 15 min. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide, and slides were incubated with rabbit monoclonal primary antibody against PD-L1 (1:200 dilution; clone E1L3N, 13684S; Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C. Slides were incubated with a secondary antibody (anti-rabbit EnVision™+/horseradish peroxidase; Dako Japan, Tokyo, Japan) and counterstained with haematoxylin (Supplementary Fig. 1).
Evaluation of PD-L1 expression
PD-L1 expressions in biopsy and resected samples were assessed by one of the investigators (K.Y.) and an experienced pathologist (Y.K.) who were unaware of the clinical data. The observers individually calculated the combined positive score (number of PD-L1-positive cells [tumour cells, macrophages and lymphocytes] divided by the total number of tumour cells, multiplied by 100) and averaged scores to establish consensus. PD-L1 positivity was defined when the average score was 1 or greater, as described previously.2 All samples included at least 100 viable tumour cells, which is the recommended tumour volume for scoring.6
Statistical analysis
To examine the consistency of PD-L1 expression between biopsy and resected samples, the kappa coefficient was calculated. The accordance rate was compared using Chi-square tests based on the number of biopsies. Statistical analyses were performed by JMP® version 13.1 software (SAS Institute). p < 0.05 was considered statistically significant.
Results
PD-L1 expression in biopsy and resected samples
Among the 191 patients, 89 (46.6%) patients showed PD-L1-positive biopsy and 135 (70.1%) patients showed PD-L1-positive resected samples (Table 1). The accordance rate of PD-L1 positivity between the biopsy and resected samples was 64.4% and the kappa coefficient value was equal to 0.31 (poor agreement). False negativity (negative PD-L1 in biopsy and positive PD-L1 in a resected specimen) was observed in 57 cases (29.8%) and was the main factor for the high discordance of PD-L1 expression between the biopsy and resected specimen.
Correlation between the number of biopsies and accordance rate
To assess the impact of the number of biopsies on the accuracy of PD-L1 evaluation, the correlation between the number of biopsies and accordance rate of PD-L1 expression was examined (Fig. 1). Among the 191 cases, single biopsy was performed in 43 cases (22.5%) and multiple biopsies were performed in 148 cases (77.5%). The accordance rate of single-biopsy cases was significantly lower (48.8%) than that of multiple biopsy cases (68.9%) (p < 0.05, Fig. 1a, b). Moreover, single-biopsy cases showed low positive and negative percent agreement (Supplementary Table 2). Multivariate logistic regression analysis demonstrated that single biopsy was associated with discordance (Supplementary Table 3). False-negative cases comprised 46% of single-biopsy cases, whereas the proportion of true-positive cases and true-negative cases increased by 10% in multiple biopsy cases (Fig. 1c).
Correlation between the number of biopsies and accordance rate. a Comparison of the accordance rate of PD-L1 expression between biopsy and resected samples according to the number of biopsies. *p < 0.05. b Comparison of the accordance rate of PD-L1 expression between biopsy and resected samples according to single or multiple biopsies. *p < 0.05. c Distribution of PD-L1 expression evaluated by biopsy according to single or multiple biopsies
Discussion
Our results showed that biopsy could not fully reflect PD-L1 expression of the whole tumour in GC. PD-L1 positivity in biopsy samples was observed in 46.6% of the GC patients, which was consistent with previous reports.2 In contrast, PD-L1 positivity was observed in 70.7% of paired resected samples. Moreover, the accordance rate of PD-L1 expression between biopsy and resected samples was 64.4%, with a marked amount of false-negative cases. Notably, a significantly lower accordance rate was observed among GC patients with single biopsy than those with multiple biopsies. Therefore, multiple biopsies are recommended to improve the accurate diagnosis of PD-L1 expression in GC.
The discordance of PD-L1 expression between biopsy and resected samples was also reported in NSCLC.7 However, the heterogeneity of PD-L1 expression specific to GC may be an important contributing factor for the discordance. Previous studies reported GC cases in which PD-L1-expressing cells localised only in the tumour centre.8 Therefore, we hypothesised that biopsy samples might not fully reflect the PD-L1 expression in the whole tumour in GC, because biopsy samples are usually obtained from the mucosal part of the tumour. Notably, we demonstrated that single biopsy is insufficient to overcome the tumour heterogeneity for the accurate assessment of PD-L1 expression. In evaluating HER2 expression in GC, several guidelines have indicated that a minimum of five biopsy specimens should be obtained to account for intratumoural heterogeneity and to provide sufficient tumour specimens for diagnosis and biomarker testing.9 We also found a higher accordance rate in cases with more than five biopsies (Supplementary Table 2). Further studies using more samples, including more than five biopsies, are needed to determine the optimal number of biopsies for PD-L1 evaluation.
This study has several limitations. First, patients who underwent pretreatment for GC were excluded. Previous clinical trials evaluated PD-L1 expression in patients with treatment history. However, PD-L1 expression can be dynamically modulated in response to treatment.10 Therefore, we excluded patients who underwent pretreatments to avoid this alteration bias. Second, we did not assess whether the discordance of PD-L1 evaluation between the biopsy and resected samples affects the prediction of the PD-1 inhibitor efficacy. Further studies are needed to confirm the significance of PD-L1 expression in the whole tumour as a predictive biomarker for PD-1 inhibitors.
In conclusion, we demonstrate that biopsy cannot fully reflect the PD-L1 expression of the whole tumour in GC. Multiple biopsies are recommended to improve accurate diagnosis of PD-L1 expression. This study is clinically meaningful in discussing the significance of PD-L1 expression as a predictive biomarker for PD-1/PD-L1 inhibitors in GC.
References
Kang, Y. K., Boku, N., Satoh, T., Ryu, M. H., Chao, Y., Kato, K. et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 2461–2471 (2017).
Fuchs, C. S., Doi, T., Jang, R. W., Muro, K., Satoh, T., Machado, M. et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4, e180013 (2018).
Gibney, G. T., Weiner, L. M. & Atkins, M. B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 17, e542–e551 (2016).
Shitara, K., Ozguroglu, M., Bang, Y. J., Di Bartolomeo, M., Mandala, M., Ryu, M. H. et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133 (2018).
Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018).
Kulangara K., Zhang N., Corigliano E., Guerrero L., Waldroup S., Jaiswal D., et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch. Pathol. Lab. Med. 143, 330–337 (2019).
Munari, E., Zamboni, G., Marconi, M., Sommaggio, M., Brunelli, M., Martignoni, G. et al. PD-L1 expression heterogeneity in non-small cell lung cancer: evaluation of small biopsies reliability. Oncotarget 8, 90123–90131 (2017).
Boger, C., Behrens, H. M., Mathiak, M., Kruger, S., Kalthoff, H. & Rocken, C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 7, 24269–24283 (2016).
Bartley, A. N., Washington, M. K., Colasacco, C., Ventura, C. B., Ismaila, N., Benson, A. B. 3rd et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 35, 446–464 (2017).
Chen, J., Jiang, C. C., Jin, L. & Zhang, X. D. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann. Oncol. 27, 409–416 (2016).
Acknowledgements
The authors thank Dr. Masaaki Iwatsuki for helping with the study; Dr. Miyake, Ms. Yasuda, Ms. Ogata and Ms. Taniguchi for their excellent technical assistance; Dr. Yuki Koga and Dr. Yuki Kiyozumi for their assistance with acquisition of data; Dr. Yoshihiro Komohara for pathological assessment; Dr. Kojiro Eto, Dr. Yukiharu Hiyoshi, Dr. Shiro Iwagami, Dr. Yoshifumi Baba, Dr. Yuji Miyamoto, Dr. Naoya Yoshida, Jaffer A. Ajani and Prof. Hideo Baba for assistance with revision of the paper. We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this paper.
Author contributions
K.Y. and M.I. performed laboratory work, collected all the samples and performed all statistical analyses. K.Y. and M.I. drafted and coordinated the writing of the paper. All authors contributed to the writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval for this study was granted by the ethical committee at Kumamoto University Hospital (Approval number: 1037). The study was conducted in accordance with the Declaration of Helsinki principles.
Funding
This work was supported in part by the following grant and foundation: Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research; grant number 16K10463 and 16KK0184.
Data availability
Individual-level data may be shared on request if all legal and ethical requirements are met. Requests should be sent to the corresponding author.
Note
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamashita, K., Iwatsuki, M., Harada, K. et al. Can PD-L1 expression evaluated by biopsy sample accurately reflect its expression in the whole tumour in gastric cancer?. Br J Cancer 121, 278–280 (2019). https://doi.org/10.1038/s41416-019-0515-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-019-0515-5
This article is cited by
-
Computed tomography-detected extramural venous invasion-related gene signature: a potential negative biomarker of immune checkpoint inhibitor treatment in patients with gastric cancer
Journal of Translational Medicine (2023)
-
Immunotherapeutic strategy in the management of gastric cancer: molecular profiles, current practice, and ongoing trials
Journal of the Egyptian National Cancer Institute (2023)
-
Preclinical development of ZED8, an 89Zr immuno-PET reagent for monitoring tumor CD8 status in patients undergoing cancer immunotherapy
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
PD-L1 CPS Scoring Accuracy in Small Biopsies and Aspirate Cell Blocks from Patients with Head and Neck Squamous Cell Carcinoma
Head and Neck Pathology (2020)