Abstract
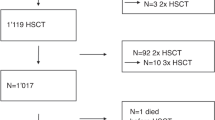
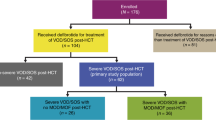
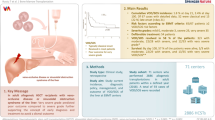
We attempted to identify the incidence and survival outcome of hepatic sinusoidal obstruction syndrome/veno-occlusive disease (VOD/SOS) after hematopoietic cell transplantation (HCT) under strategy of prophylactic ursodiol and intravenous heparin or prostaglandin E1 (PGE1). From 2009 to 2018, 2572 consecutive allogeneic-HCT cases were reviewed. We used oral ursodiol for all transplants, and most were administered low-dose heparin, while PGE1 in selected cases with low platelet count at the time of preconditioning. Diagnosis and severity grades were reassessed by revised EBMT criteria. The overall incidence of hepatic VOD/SOS was 3.4% (Mild 0.9%, Moderate 0.6%, Severe 0.7%, Very severe 1.2%) after allogeneic-HCT under strategy of intravenous prophylaxis. The 1-year overall survival of VOD/SOS was 41.4% which was divided into 73.9% for mild, 66.7% for moderate, 38.9% for severe, and 6.5% for very severe grade. Very high disease risk index, male gender, donor other than matched sibling donor, and busulfex > 9 mg/kg were affecting factors for development of VOD/SOS. For severe to very severe VOD/SOS, history of pre-HCT liver dysfunction was an additionally affecting factor. Allogeneic-HCT using ursodiol and intravenous prophylaxis was considered safe without significant bleeding complications and should be evaluated in future clinical trials. For those with high-risk of VOD/SOS, early intervention and management is important.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Yoon JH, Yoo KH, Sung KW, Jung CW, Kim JS, Hahn SM, et al. Validation of treatment outcomes according to revised severity criteria from European Society for Blood and Marrow Transplantation (EBMT) for sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD). Bone Marrow Transpl. 2019;54:1361–8. https://doi.org/10.1038/s41409-019-0492-6
Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transpl. 2010;16:157–68. https://doi.org/10.1016/j.bbmt.2009.08.024
Lee SH, Yoo KH, Sung KW, Koo HH, Kwon YJ, Kwon MM, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transpl. 2010;45:1287–93. https://doi.org/10.1038/bmt.2009.349
Yoon JH, Min WS, Kim HJ, Kim JH, Shin SH, Yahng SA, et al. Experiences of t-PA use in moderate-to-severe hepatic veno-occlusive disease after hematopoietic SCT: is it still reasonable to use t-PA? Bone Marrow Transpl. 2013;48:1562–8. https://doi.org/10.1038/bmt.2013.101
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67.
Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003;10:451–62.
Carreras E, Bertz H, Arcese W, Vernant JP, Tomas JF, Hagglund H, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599–604.
Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–9. https://doi.org/10.1016/S0140-6736(11)61938-7
Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transpl. 2018;53:138–45. https://doi.org/10.1038/bmt.2017.161
Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. 2005;90:1396–404. e-pub ahead of print 2005/10/13
Tsirigotis PD, Resnick IB, Avni B, Grisariu S, Stepensky P, Or R, et al. Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transpl. 2014;49:1389–92. https://doi.org/10.1038/bmt.2014.168
Carreras E, Diaz-Beya M, Rosinol L, Martinez C, Fernandez-Aviles F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transpl. 2011;17:1713–20. https://doi.org/10.1016/j.bbmt.2011.06.006
Ramasamy K, Lim ZY, Pagliuca A, Grundy R, Devereux S, Ho AY, et al. Incidence and management of hepatic venoocclusive disease in 237 patients undergoing reduced-intensity conditioning (RIC) haematopoietic stem cell transplantation (HSCT). Bone Marrow Transpl. 2006;38:823–4. https://doi.org/10.1038/sj.bmt.1705528
Radich JP, Sanders JE, Buckner CD, Martin PJ, Petersen FB, Bensinger W, et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol. 1993;11:304–13. https://doi.org/10.1200/JCO.1993.11.2.304
Marks DI, Kebriaei P, Stelljes M, Gokbuget N, Kantarjian H, Advani AS, et al. Outcomes of allogeneic stem cell transplantation after inotuzumab ozogamicin treatment for relapsed or refractory acute lymphoblastic leukemia. Biol Blood Marrow Transpl. 2019;25:1720–9. https://doi.org/10.1016/j.bbmt.2019.04.020
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab Ozogamicin versus standard therapy for acute lymphoblastic leukemia. N. Engl J Med. 2016;375:740–53. https://doi.org/10.1056/NEJMoa1509277
Simon M, Hahn T, Ford LA, Anderson B, Swinnich D, Baer MR, et al. Retrospective multivariate analysis of hepatic veno-occlusive disease after blood or marrow transplantation: possible beneficial use of low molecular weight heparin. Bone Marrow Transpl. 2001;27:627–33. https://doi.org/10.1038/sj.bmt.1702854
Imran H, Tleyjeh IM, Zirakzadeh A, Rodriguez V, Khan SP. Use of prophylactic anticoagulation and the risk of hepatic veno-occlusive disease in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Bone Marrow Transpl. 2006;37:677–86. https://doi.org/10.1038/sj.bmt.1705297
Gluckman E, Jolivet I, Scrobohaci ML, Devergie A, Traineau R, Bourdeau-Esperou H, et al. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br J Haematol. 1990;74:277–81. https://doi.org/10.1111/j.1365-2141.1990.tb02583.x
Attal M, Huguet F, Rubie H, Huynh A, Charlet JP, Payen JL, et al. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood. 1992;79:2834–40.
Tay J, Tinmouth A, Fergusson D, Huebsch L, Allan DS. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2007;13:206–17. https://doi.org/10.1016/j.bbmt.2006.09.012
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. https://doi.org/10.1182/blood-2014-01-552984
Yoon JH, Min GJ, Park SS, Jeon YW, Lee SE, Cho BS, et al. Minimal residual disease-based long-term efficacy of reduced-intensity conditioning versus myeloablative conditioning for adult Philadelphia-positive acute lymphoblastic leukemia. Cancer. 2019;125:873–83. https://doi.org/10.1002/cncr.31874
Lee SE, Kim HJ, Min WS, Cho BS, Eom KS, Kim YJ, et al. Favorable outcomes of intravenous busulfan, fludarabine, and 400 cGy total body irradiation-based reduced-intensity conditioning allogeneic stem cell transplantation for acute myelogenous leukemia with old age and/or co-morbidities. Int J Hematol. 2010;92:342–50. https://doi.org/10.1007/s12185-010-0649-y
Lee SE, Kim YJ, Yahng SA, Cho BS, Eom KS, Lee S, et al. Survival benefits from reduced-intensity conditioning in allogeneic stem cell transplantation for young lower-risk MDS patients without significant comorbidities. Eur J Haematol. 2011;87:510–20. https://doi.org/10.1111/j.1600-0609.2011.01697.x
Shin SH, Jeon YW, Yoon JH, Yahng SA, Lee SE, Cho BS, et al. Comparable outcomes between younger (40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transpl. 2016;51:1456–63. https://doi.org/10.1038/bmt.2016.171
Lee SE, Park SS, Jeon YW, Yoon JH, Cho BS, Eom KS, et al. Optimal conditioning regimen for haplo-identical stem cell transplantation in adult patients with acquired severe aplastic anemia: prospective de-escalation study of TBI and ATG dose. Am J Hematol. 2018;93:1368–75. https://doi.org/10.1002/ajh.25257
McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116–22.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83. e-pub ahead of print 1987/12/01
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2015;50:781–9. https://doi.org/10.1038/bmt.2015.52
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transpl. 2016;51:906–12. https://doi.org/10.1038/bmt.2016.130
Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656–65. https://doi.org/10.1182/blood-2015-10-676924
Richardson PG, Carreras E, Iacobelli M, Nejadnik B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018;2:1495–509. https://doi.org/10.1182/bloodadvances.2017008375
Richardson PG, Triplett BM, Ho VT, Chao N, Dignan FL, Maglio M, et al. Defibrotide sodium for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Exp Rev Clin Pharmacol. 2018;11:113–24. https://doi.org/10.1080/17512433.2018.1421943
Kim DS. Introduction: health of the health care system in Korea. Soc Work Public Health. 2010;25:127–41. https://doi.org/10.1080/19371910903070333
Yakushijin K, Atsuta Y, Doki N, Yokota A, Kanamori H, Miyamoto T, et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transpl. 2016;51:403–9. https://doi.org/10.1038/bmt.2015.283
Essell JH, Schroeder MT, Harman GS, Halvorson R, Lew V, Callander N, et al. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128(12 Pt 1):975–81. https://doi.org/10.7326/0003-4819-128-12_part_1-199806150-00002
Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–83. https://doi.org/10.1182/blood-2001-12-0159
Ohashi K, Tanabe J, Watanabe R, Tanaka T, Sakamaki H, Maruta A, et al. The Japanese multicenter open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol. 2000;64:32–38. https://doi.org/10.1002/(sici)1096-8652(200005)64:1<32::aid-ajh6>3.0.co;2-n
Park SH, Lee MH, Lee H, Kim HS, Kim K, Kim WS, et al. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2002;29:137–43. https://doi.org/10.1038/sj.bmt.1703342
Marsa-Vila L, Gorin NC, Laporte JP, Labopin M, Dupuy-Montbrun MC, Fouillard L, et al. Prophylactic heparin does not prevent liver veno-occlusive disease following autologous bone marrow transplantation. Eur J Haematol. 1991;47:346–54. https://doi.org/10.1111/j.1600-0609.1991.tb01859.x
Or R, Nagler A, Shpilberg O, Elad S, Naparstek E, Kapelushnik J, et al. Low molecular weight heparin for the prevention of veno-occlusive disease of the liver in bone marrow transplantation patients. Transplantation. 1996;61:1067–71. https://doi.org/10.1097/00007890-199604150-00014
Bearman SI, Shen DD, Hinds MS, Hill HA, McDonald GB. A phase I/II study of prostaglandin E1 for the prevention of hepatic venocclusive disease after bone marrow transplantation. Br J Haematol. 1993;84:724–30. https://doi.org/10.1111/j.1365-2141.1993.tb03152.x
Song JS, Seo JJ, Moon HN, Ghim T, Im HJ. Prophylactic low-dose heparin or prostaglandin E1 may prevent severe veno-occlusive disease of the liver after allogeneic hematopoietic stem cell transplantation in Korean children. J Korean Med Sci. 2006;21:897–903. https://doi.org/10.3346/jkms.2006.21.5.897
Richardson PG, Smith AR, Triplett BM, Kernan NA, Grupp SA, Antin JH, et al. Earlier defibrotide initiation post-diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome improves Day +100 survival following haematopoietic stem cell transplantation. Br J Haematol. 2017;178:112–8. https://doi.org/10.1111/bjh.14727
Richardson PG, Smith AR, Triplett BM, Kernan NA, Grupp SA, Antin JH, et al. Defibrotide for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome: interim results from a treatment IND study. Biol Blood Marrow Transpl. 2017;23:997–1004. https://doi.org/10.1016/j.bbmt.2017.03.008
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
Written informed consent was obtained at the stage of transplantation in all patients.
Ethics approval and consent to participate
This research was conducted in accordance with the Institutional Review Board guidelines of the Catholic Medical Center (KC20RISI0132/199).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yoon, JH., Min, G.J., Park, SS. et al. Incidence and risk factors of hepatic veno-occlusive disease/sinusoidal obstruction syndrome after allogeneic hematopoietic cell transplantation in adults with prophylactic ursodiol and intravenous heparin or prostaglandin E1. Bone Marrow Transplant 56, 1603–1613 (2021). https://doi.org/10.1038/s41409-021-01215-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01215-y
This article is cited by
-
Prevention of veno-occlusive disease/sinusoidal obstruction syndrome: a never-ending story and no easy answer
Bone Marrow Transplantation (2023)
-
Low Incidence of hepatic sinusoidal obstruction syndrome/veno-occlusive disease in adults undergoing allogenic stem cell transplantation with prophylactic ursodiol and low-dose heparin
Bone Marrow Transplantation (2022)
-
Prediction and recommendation by machine learning through repetitive internal validation for hepatic veno-occlusive disease/sinusoidal obstruction syndrome and early death after allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2022)
-
Identification of an asymptomatic Shwachman–Bodian–Diamond syndrome mutation in a patient with acute myeloid leukemia
International Journal of Hematology (2022)
-
The association between serum sodium level and tuberculous meningitis compared with viral and bacterial meningitis
Scientific Reports (2021)