Abstract
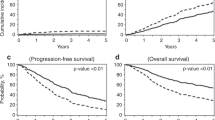
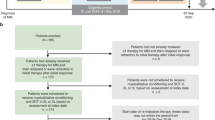
We conducted a retrospective study comparing posttransplant outcomes between myeloma patients receiving conditioning melphalan on day-2 vs day-1 for autologous stem cell transplant. Between January 2017 and December 2018, 201 patients received melphalan on day-2 and 166 on day-1 prior to stem cell infusion. Baseline disease and clinical characteristics between the two groups were similar. Although rates of hospitalization were similar between the cohorts, duration of hospital admission was longer for day-1 (median 7 days for day-1 vs 5 days for day-2, p = 0.003). Rates of fever were higher in the day-1 cohort (69% vs 49%, p = 0.0002). Time to platelet and neutrophil engraftment was significantly longer in the day-1 cohort (platelet engraftment median days 17 for day-1 vs 15 for day-2, p < 0.0001, neutrophil engraftment median days 16 for day-1 vs 16 for day-2, p = 0.025). Overall response rate was similar between the cohorts (99% for day-1, vs 100% for day-2). Day-2 melphalan infusions should be considered in preference for day-1 protocols, given the clinically significant delay in platelet and neutrophil engraftment and longer duration of hospitalization with day-1 infusions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl J Med. 2003;348:1875–83.
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–5.
Anagnostopoulos A, Aleman A, Ayers G, Donato M, Champlin R, Weber D, et al. Comparison of high-dose melphalan with a more intensive regimen of thiotepa, busulfan, and cyclophosphamide for patients with multiple myeloma. Cancer. 2004;100:2607–12.
Fenk R, Schneider P, Kropff M, Huenerlituerkoglu AN, Steidl U, Aul C, et al. High-dose idarubicin, cyclophosphamide and melphalan as conditioning for autologous stem cell transplantation increases treatment-related mortality in patients with multiple myeloma: results of a randomised study. Br J Haematol. 2005;130:588–94.
Palumbo A, Bringhen S, Bruno B, Falcone AP, Liberati AM, Grasso M, et al. Melphalan 200 mg/m(2) versus melphalan 100 mg/m(2) in newly diagnosed myeloma patients: a prospective, multicenter phase 3 study. Blood. 2010;115:1873–9.
Samuels BL, Bitran JD. High-dose intravenous melphalan: a review. J Clin Oncol. 1995;13:1786–99.
Alberts DS, Chang SY, Chen HS, Moon TE, Evans TL, Furner RL, et al. Kinetics of intravenous melphalan. Clin Pharm Ther. 1979;26:73–80.
Mahindra A, Bolwell B, Rybicki L, Sobecks R, Pohlman B, Andresen S, et al. Timing of high dose melphalan (HDM) and outcomes for autologous stem cell transplantation (ASCT) in patients with multiple myeloma (MM). Biol Blood Marrow Transplant. 2009;15:41–2.
Talamo G, Rakszawski KL, Rybka WB, Dolloff NG, Malysz J, Berno T, et al. Effect of time to infusion of autologous stem cells (24 vs. 48 h) after high-dose melphalan in patients with multiple myeloma. Eur J Haematol. 2012;89:145–50.
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–29.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N. Engl J Med. 2014;371:895–905.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33:2863–9.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46.
Acknowledgements
The study was approved by the Mayo Clinic institutional review board.
Funding
NCI SPORE MM SPORE 5P50 CA186781-04.
Author information
Authors and Affiliations
Contributions
ASA, MHS designed the study, analyzed the data, wrote the first draft, and approved the final version of the paper. JL, EJ, CAS, SKW collected the data, revised the paper and approved the final version of the paper AD, EM, FKB, RW, MQL, DD, NL, WIG, PK, TVK, WJH, RW, and SKK performed patient management, revised the paper critically, and approved the final version of the paper; and MAG designed the study, analyzed the data, wrote the first draft, approved the final version of the paper, and performed patient management.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Saleh, A.S., Sidiqi, M.H., Lee, J. et al. Differences in engraftment with day-1 compared with day-2 melphalan prior to stem cell infusion in myeloma patients receiving autologous stem cell transplant. Bone Marrow Transplant 55, 2132–2137 (2020). https://doi.org/10.1038/s41409-020-0916-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0916-3
This article is cited by
-
Melphalan pharmacokinetics and transplant related toxicity during autologous stem cell transplantation
Bone Marrow Transplantation (2021)
-
Still learning the right way to administer melphalan in autologous transplantation
Bone Marrow Transplantation (2020)