Abstract
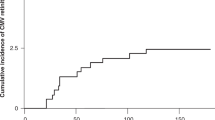
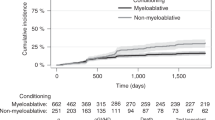
This study investigated the epidemiological characteristics of cytomegalovirus retinitis (CMVR) after haploidentical hematopoietic stem cell transplantation (HSCT). We studied a cohort of 1466 consecutive patients who had undergone haploidentical HSCT between 2013 and 2017. We documented 34 episodes of CMVR in 31 patients, with a median onset of 167 days after the transplant. The cumulative incidence of CMVR was 2.3% 1 year after the transplant. Multivariate analysis suggested that platelet engraft failure at 100 days, EBV DNAemia, refractory or recurrent CMV DNAemia, and acute graft-versus-host disease were related to the development of CMVR in patients with CMV DNAemia. Patients with ≥3 risk factors (high risk) had a higher 1-year incidence of CMVR than patients with ≤2 risk factors (low risk) (26.2% vs. 0.6%, P < 0.001). In patients with CMVR, visual acuity (VA) improved in 16 episodes, remained stable in 10 episodes, and worsened in 8 episodes. The variable related to the improvement of VA was VA ≥ 0.1 at time of CMVR diagnosis. Our study showed that CMVR was a rare complication after haploidentical HSCT but that the risk was greater in patients with multiple risk factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eid AJ, Bakri SJ, Kijpittayarit S, Razonable RR. Clinical features and outcomes of cytomegalovirus retinitis after transplantation. Transpl Infect Dis. 2008;10:13–18.
Hiwarkar P, Gajdosova E, Qasim W, Worth A, Breuer J, Chiesa R, et al. Frequent occurrence of cytomegalovirus retinitis during immune reconstitution warrants regular ophthalmic screening in high-risk pediatric allogeneic hematopoietic stem cell transplantrecipients. Clin Infect Dis. 2014;58:1700–6.
Xhaard A, Robin M, Scieux C, de Latour RP, Deplus S, Mazeron MC, et al. Increased incidence of cytomegalovirus retinitis after allogeneic hematopoietic stem cell transplantation. Transplantation. 2007;83:80–83.
Jeon S, Lee WK, Lee Y, Lee DG, Lee JW. Risk factors for cytomegalovirus retinitis in patients with cytomegalovirus viremia after hematopoietic stem cell transplantation. Ophthalmology. 2012;119:1892–1298.
Crippa F, Corey L, Chuang EL, Sale G, Boeckh M. Virological, clinical, and ophthalmologic features of cytomegalovirus retinitis after hematopoietic stem cell transplantation. Clin Infect Dis. 2001;32:214–9.
Maury S, Mary JY, Rabian C, Schwarzinger M, Toubert A, Scieux C, et al. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br J Haematol. 2001;115:630–41.
Janeczko M, Mielcarek M, Rybka B, Ryczan-Krawczyk R, Noworolska-Sauren D, Kałwak K. Immune recovery and the risk of CMV/EBV reactivation in children post allogeneic haematopoietic stem cell transplantation. Cent Eur J Immunol. 2016;41:287–96.
Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transpl. 2017;52:811–7.
Xu LP, Wu DP, Han MZ, Huang H, Liu QF, Liu DH, et al. A review of hematopoietic cell transplantation in China: data and trends during 2008-2016. Bone Marrow Transpl. 2017;52:1512–8.
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–73.
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–3862.
Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30:2055–63.
Chang YJ, Zhao XY, Huang XJ. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2014;20:440–9.
Chang YJ, Zhao XY, Huo MR, Xu LP, Liu DH, Liu KY, et al. Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. J Clin Immunol. 2012;32:268–80.
Huang JJ, Lu XQ, Yan CH, Zhao XS, Xu LP, Huang XJ, et al. Comparative study on clinical features of cytomegalovirus infection after allogenic hematopoietic stem cell transplantation from HLA haploidentical related donors vs HLA-matched sibling donors. Chin J Organ Transpl. 2013;34:87–91.
Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk diseasefollowing haploidentical transplantation: a randomized trial. Bone Marrow Transpl. 2014;49:426–33.
Tomas ED, Storb R, Clift RA, Fefer A, Johnson L, Neiman PE, et al. Bone marrow transplantation. N. Engl J Med. 1975;292:895–902.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–56.
Pei XY, Zhao XY, Chang YJ, Liu J, Xu LP, Wang Y, et al. Cytomegalovirus-specific T-Cell transfer for refractory cytomegalovirus infection after haploidentical stem cell transplantation: the quantitative and qualitative immune recovery for cytomegalovirus. J Infect Dis. 2017;216:945–56.
Liu J, Kong J, Chang YJ, Chen H, Chen YH, Han W, et al. Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoieticstem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin Microbiol Infect. 2015;21:1121. e9-15
Miao H, Hou J. Influent factors for treating procedure of cytomegalovirus retinitis after allogeneic bone marrow hematopoietic stem cell transplantation. Chin J Ophthalmol 2017;53:740–5.
Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91.
Chemaly RF, Chou S, Einsele H, Griffiths P, Avery R, Razonable RR, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. 2019;68:1420–6.
Kong Y, Wang YT, Cao XN, Song Y, Chen YH, Sun YQ, et al. Aberrant T cell responses in the bone marrow microenvironment of patients with poor graft function after allogeneic hematopoietic stem cell transplantation. J Transl Med. 2017;15:57.
Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–14.
Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–7.
Lilleri D, Fornara C, Chiesa A, Caldera D, Alessandrino EP, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in adult allogeneic hematopoietic stem cell transplant recipients and immune control of viral infection. Haematologica. 2008;93:248–56.
Liu J, Chang YJ, Yan CH, Xu LP, Jiang ZF, Zhang XH, et al. Poor CMV-specific CD8+ T central memory subset recovery at early stage post-HSCT associates with refractory and recurrent CMV reactivation. J Infect. 2016;73:261–70.
Gratama JW, Brooimans RA, van der Holt B, Sintnicolaas K, van Doornum G, Niesters HG, et al. Monitoring cytomegalovirus IE-1 and pp65-specific CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation may identify patients at risk for recurrent CMV reactivations. Cytom B Clin Cytom. 2008;74:211–20.
Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneicbone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–9.
Gratama JW, van Esser JW, Lamers CH, Tournay C, Löwenberg B, Bolhuis RL, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98:1358–64.
Cwynarski K, Ainsworth J, Cobbold M, Wagner S, Mahendra P, Apperley J, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97:1232–40.
Sinclair E, Tan QX, Sharp M, Girling V, Poon C, Natta MV, et al. Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon- gamma and interleukin-2 and have a CD8+ cell early maturational phenotype. J Infect Dis. 2006;194:1537–46.
Ljungman P, Perez-Bercoff L, Jonsson J, Avetisyan G, Sparrelid E, Aschan J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91:78–83.
Wu GJ, Chen SZ, Chen LY, Yao MH, Dai G. Study on molecular epidemiology of HCMV infection in mothers and their newborns in Changsha. Bull Hunan Med Univ. 2001;26:23–25.
Zhang Y, Ruan X, Yang W, Li L, Xian Z, Feng Q, et al. High ocular CMV copies and mismatched receipts may predict poor visual prognosis in CMV retinitis patients following allogeneic haematopoietic stem cell transplantation. BMC Ophthalmol. 2017;17:224–31.
Wei LL, Park SS, Skiest DJ. Prevalence of visual symptoms among patients with newly diagnosed cytomegalovirus retinitis. Retina. 2002;22:278–82.
Streilein J. Regional immunology of the eye: ocular infection and immunology. St. Louis: CV Mosby; 1996. p. 19–32.
Kuppermann BD, Quiceno JI, Flores-Aguilar M, Connor JD, Capparelli EV, Sherwood CH, et al. Intravitreal ganciclovir concentration after intravenous administration in AIDS patients with cytomegalovirus retinitis: implications for therapy. J Infect Dis. 1993;168:1506–9.
Funding
This study was supported by the National Natural Science Foundation of China (Grant no. 81400142). The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
X-JH participated in the research design. C-HY participated in the writing of the manuscript and data analysis. All other authors participated in providing patient data. Yi-lin Yuan, Jia-xiang Gao, and Shi-ning Fu participated in collecting patient information.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yan, CH., Wang, Y., Mo, Xd. et al. Incidence, risk factors, and outcomes of cytomegalovirus retinitis after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant 55, 1147–1160 (2020). https://doi.org/10.1038/s41409-020-0790-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0790-z
This article is cited by
-
Posterior segment complications and the risk factors after allogeneic hematopoietic stem cell transplantation
Eye (2023)
-
Risk factors and outcomes of diffuse alveolar haemorrhage after allogeneic haematopoietic stem cell transplantation
Bone Marrow Transplantation (2021)
-
Comparison of different cytomegalovirus diseases following haploidentical hematopoietic stem cell transplantation
Annals of Hematology (2020)