Abstract
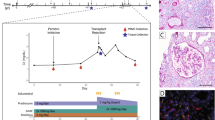
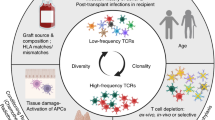
This review focuses on our recent studies involving nonmyeloablative bone marrow transplantation as an approach to inducing organ allograft tolerance across MHC barriers in nonhuman primates and in patients. The clinical studies are focused on mechanisms of tolerance involved in a protocol carried out at Massachusetts General Hospital in HLA-mismatched haploidentical combinations for the induction of renal allograft tolerance. These studies, in which chimerism was only transient and GVHD did not occur, suggest an early role for donor-specific regulatory T cells in tolerance induction, followed by partial and gradual deletion of donor-reactive T cells. We utilized high-throughput sequencing methodologies in a novel way to identify and track large numbers of alloreactive T cell receptors (TCRs). This method has been shown to identify biologically significant alloreactive TCRs in transplant patients and pointed to clonal deletion as a major mechanism of long-term tolerance in these patients. More recently, we adapted this sequencing method to optimally identify the donor-specific regulatory T cell (Treg) repertoire. Interrogation of the early posttransplant repertoire demonstrated expansion of donor-specific Tregs in association with tolerance. Our studies suggest a role for the kidney graft in tolerance by these mechanisms in patients who had only transient chimerism. Nonhuman primate studies indicate that other organs, including the heart, the lungs and the liver, are less readily tolerated following a period of transient mixed chimerism. Our efforts to extend the reach of mixed chimerism for tolerance induction beyond the kidney are therefore focused on the addition of recipient Tregs to the protocol. This approach has the potential to enhance chimerism while further reducing the risk of GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. New Engl J Med. 2008;358:353–61.
Kawai T, Sachs DH, Sykes M, Cosimi AB, Immune Tolerance N. HLA-mismatched renal transplantation without maintenance immunosuppression. New Engl J Med. 2013;368:1850–2.
Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502.
Tomita Y, Khan A, Sykes M. Mechanism by which additional monoclonal antibody injections overcome the requirement for thymic irradiation to achieve mixed chimerism in mice receiving bone marrow transplantation after conditioning with anti-T cell mAbs and 3 Gy whole body irradiation. Transplantation. 1996;61:477–85.
Tomita Y, Sachs DH, Khan A, Sykes M. Additional mAb injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti-T cell mAbs and 3 Gy whole body irradiation. Transplantation. 1996;61:469–77.
Nikolic B, Khan A, Sykes M. Induction of tolerance by mixed chimerism with nonmyeloblative host conditioning: the importance of overcoming intrathymic alloresistance. Biol Blood Marrow Transplant. 2001;7:144–53.
Sykes M, Sheard MA, Sachs DH. Graft-versus-host-related immunosuppression is induced in mixed chimeras by alloresponses against either host or donor lymphohematopoietic cells. J Exp Med. 1988;168:2391–6.
Mapara MY, Kim Y-M, Wang S-P, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100:1903–9.
Mapara MY, Kim Y-M, Marx J, Sykes M, DLI-mediated GVL. effects in mixed chimeras established with a non-myeloablative conditioning regimen: extinction of GVL effects coincides with loss of alloreactive cells following conversion to full donor chimerism. Transplantation. 2003;76:297–305.
Pelot MR, Pearson DA, Swenson K, Zhao G, Sachs J, Yang Y-G, et al. Lymphohematopoietic graft-vs-host reactions can be induced without graft-vs-host disease in murine mixed chimeras established with a cyclophosphamide-based non-myeloablative conditioning regimen. Biol Blood Marrow Transplant. 1999;5:133–43.
Mapara MY, Leng C, Kim YM, Bronson R, Lokshin A, Luster A, et al. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol Blood Marrow Transplant. 2006;12:623–34.
Chakraverty R, Cote D, Buchli J, Cotter P, Hsu R, Zhao G, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host-reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–31.
Sykes M, Preffer F, McAffee S, Saidman SL, Colby C, Sackstein R, et al. Mixed lymphohematopoietic chimerism and graft-vs-lymphoma effects are achievable in adult humans following non-myeloablative therapy and HLA-mismatched donor bone marrow transplantation. Lancet. 1999;353:1755–9.
Toh HC, Spitzer TR, Preffer F, Alexander SI, MCafee S, Dombkowski D, et al. Fluctuating lymphocyte chimerism, tolerance and anti-tumor response in a patient with refractory lymphoma receiving non-myeloablative conditioning an a haploidentical related allogeneic bone marrow transplant. Cytokines Cell Mol Ther. 2002;7:43–7.
Spitzer TR, MCafee S, Sackstein R, Colby C, Toh HC, Multani P, et al. The intentional induction of mixed chimerism and achievement of anti-tumor responses following non-myeloablative conditioning therapy and HLA-matched and mismatched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant. 2000;6:309–20.
Dey BR, MCafee S, Sackstein R, Colby C, Saidman S, Weymouth D, et al. Successful allogeneic stem cell transplantation with nonmyeloablative conditioning in patients with relapsed hematologic malignancy following autologous stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:604–12.
Dey BR, MCafee S, Colby C, Sackstein R, Saidman S, Tarbell N, et al. Impact of prophlactic donor leukocyte infusions on mixed chimerism, graft-vs-host disease and anti-tumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–9.
Dey BR, MCafee S, Colby C, Cieply K, Caron M, Saidman S, et al. Anti-tumor response despite loss of donor chimerism in patients treated with nonmyeloablative conditioning and allogeneic stem cell transplantation. Br J Haematol. 2005;128:351–9.
Spitzer TR, MCafee S, Dey BR, Colby C, Hope J, Grossberg H, et al. Non-myeloablative haploidentical stem cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1748–51.
Chakraverty R, Eom HS, Sachs J, Buchli J, Cotter P, Hsu R, et al. Host MHC Class II+antigen-presenting cells and CD4 cells are required for CD8-mediated graft-versus-leukemia responses following delayed donor leukocyte infusions. Blood. 2006;108:2106–13.
Chakraverty R, Flutter B, Fallah-Arani F, Eom HS, Means T, Andreola G, et al. The host environment regulates the function of CD8+ graft-versus-host-reactive effector cells. J Immunol. 2008;181:6820–8.
Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering GVHD and GVL. Blood. 2007;110:9–17.
Childs R, Clave E, Contenin N, Jayasekera D, Hensel N, Leitman S, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–41.
Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomologous monkeys. Transplantation. 1995;59:256–62.
Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–8.
Buhler LH, Spitzer TR, Sykes M, Sachs DH, Delmonico FL, Rubin-Tolkoff N, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405–9.
Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6:2121–33.
Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011;91:672–6.
Rubio MT, Kim YM, Sachs T, Mapara M, Zhao G, Sykes M. Anti-tumor effect of donor marrow graft rejection induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: critical role for recipient-derived IFN-{gamma}. Blood. 2003;102:2300–7.
Rubio MT, Saito TI, Kattelman K, Zhao G, Buchli J, Sykes M. Mechanisms of the anti-tumor responses and host-versus graft reactions induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: a critical role for recipient CD4+ T cells and recipient leukocyte infusion-derived IFN-gamma-producing CD8+ T cells. J Immunol. 2005;175:665–76.
Rubio MT, Zhao G, Buchli J, Chittenden M, Sykes M. Role of indirect allo- and autoreactivity in anti-tumor responses induced by recipient leukocyte infusions (RLI) in mixed chimeras prepared with nonmyeloablative conditioning. Clin Immunol. 2006;120:33–44.
Saito TI, Rubio MT, Sykes M. Clinical relevance of recipient leukocyte infusion as antitumor therapy following nonmyeloablative allogeneic hematopoietic cell transplantation. Exp Hematol. 2006;34:1271–7.
Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–611.
Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011;11:1236–47.
Morris HDW, DeWolf S, Robins H, Sprangers B, Locascio SA, Shonts B, et al. Tracking donor-reactive T cells: evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med. 2015. https://doi.org/10.1126/scitranslmed.3010760.
Bonnefoix T, Bonnefoix P, Mi JQ, Lawrence JJ, Sotto JJ, Leroux D. Detection of suppressor T lymphocytes and estimation of their frequency in limiting dilution assays by generalized linear regression modeling. J Immunol. 2003;170:2884–94.
Bonnefoix T, Bonnefoix P, Perron P, Mi JQ, Ng WF, Lechler R, et al. Quantitating effector and regulatory T lymphocytes in immune responses by limiting dilution analysis modeling. J Immunol. 2005;174:3421–31.
Shaffer J, Villard J, Means TK, Alexander S, Dombkowski D, Dey BR, et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp Hematol. 2007;35:1140–52.
Sprangers B, DeWolf S, Savage TM, Morokata T, Obradovic A, LoCascio SA, et al. Origin of enriched regulatory T cells in patients receiving combined kidney-bone marrow transplantation to induce transplantation tolerance. Am J Transplant. 2017;17:2020–32.
Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911.
Zuber J, Shonts B, Lau SP, Obradovic A, Fu J, Yang S, et al. Bidirectional intragraft alloreactivity drives the repopulation of human intestinal allografts and correlates with clinical outcome. Sci Immunol. 2016. https://doi.org/10.1126/sciimmunol.aah3732.
DeWolf S, Grinshpun B, Savage T, Lau SP, Obradovic A, Shonts B, et al. Quantifying size and diversity of the human T cell alloresponse. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.121256.
Savage TM, Shonts B, Obradovic A, Dewolf S, Lau S, Zuber J, et al. Early expansion of donor-specific Tregs in tolerant kidney transplant recipients. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.124086.
Aoyama A, Ng CY, Millington TM, Boskovic S, Murakami T, Wain JC, et al. Comparison of lung and kidney allografts in induction of tolerance by a mixed-chimerism approach in cynomolgus monkeys. Transplant Proc. 2009;41:429–30.
Kawai T, Cosimi AB, Wee SL, Houser S, Andrews D, Sogawa H, et al. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation. 2002;73:1757–64.
Kawai T, Sachs DH, Cosimi AB. Tolerance to vascularized organ allografts in large animal models. Curr Opin Immunol. 1999;11:516–26.
Oura T, Ko DS, Boskovic S, O’Neil JJ, Chipashvili V, Koulmanda M, et al. Kidney versus islet allograft survival after induction of mixed chimerism with combined donor bone marrow transplantation. Cell Transpl. 2016;25:1331–41.
Tonsho M, Lee S, Aoyama A, Boskovic S, Nadazdin O, Capetta K, et al. Tolerance of lung allografts achieved in nonhuman primates via mixed hematopoietic chimerism. Am J Transplant. 2015;15:2231–9.
Pilat N, Baranyi U, Klaus C, Jaeckel E, Mpofu N, Wrba F, et al. Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am J Transplant. 2010;10:751–62.
Duran-Struuck R, Sondermeijer HP, Buhler L, Alonso-Guallart P, Zitsman J, Kato Y, et al. Effect of ex vivo-expanded recipient regulatory T cells on hematopoietic chimerism and kidney allograft tolerance across MHC barriers in cynomolgus macaques. Transplantation. 2017;101:274–83.
Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767–75.
Alonso-Guallart P, Zitsman JS, Stern J, Kofman SB, Woodland D, Ho SH et al. Characterization, biology, and expansion of regulatory T cells in the Cynomolgus macaque for pre-clinical studies. Am J Transplant. 2019; https://doi.org/10.1111/ajt.15313.
Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008;8:1639–51.
Li W, Lu L, Wang Z, Wang L, Fung JJ, Thomson AW, et al. Costimulation blockade promotes the apoptotic death of graft- infiltrating T cells and prolongs survival of hepatic allografts from FLT3L-treated donors. Transplantation. 2001;72:1423–32.
Starzl TE, Murase N, Thomson A, Demetris AJ. Liver transplants contribute to their own success. Nat Med. 1996;2:163–5.
Dangi A, Sumpter TL, Kimura S, Stolz DB, Murase N, Raimondi G, et al. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188:3667–77.
Kato Y, Griesemer AD, Wu A, Sondermeijer HP, Weiner JI, Duran-Struuck R, et al. Novel H-shunt venovenous bypass for liver transplantation in cynomolgus macaques. Comp Med. 2017;67:436–41.
Chaudhry S, Emond J, Griesemer A. Immune cell trafficking to the liver. Transplantation. 2019. https://doi.org/10.1097/TP.0000000000002690.
Acknowledgements
The authors thank Nicole Casio for assistance with the manuscript.
Funding
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award UM1 AI109565. The study was also supported by NIAID grants RO1 AI084074 and PO1 AI106697, the ITN grant NO1 AI015416, and by PO1 CA111519. NHP studies were supported by NIH grants RO1 OD017949 and R56 AI122332. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Publication of this supplement was sponsored by Gilead Sciences Europe Ltd, Cell Source, Inc., The Chorafas Institute for Scientific Exchange of the Weizmann Institute of Science, Kiadis Pharma, Miltenyi Biotec, Celgene, Centro Servizi Congressuali, Almog Diagnostic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MS is chairman of the scientific advisory board of ITB-MED AB. MS serves as Scientific Advisory Board member and owns equity in Magenta Therapeutics, and received grant support from United Therapeutics, Lung Biotechnology, ITB-Med AB. Additional co-author has declared that no conflict of interest exists.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sykes, M., Griesemer, A.D. Transplantation tolerance in nonhuman primates and humans. Bone Marrow Transplant 54 (Suppl 2), 815–821 (2019). https://doi.org/10.1038/s41409-019-0620-3
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0620-3