Abstract
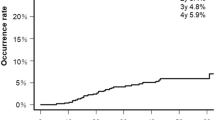
Allogeneic hematopoietic stem cell transplantation is the only curative option for a variety of diseases. Despite advances, it is associated with considerable morbidity and mortality, often involving liver complications. Liver disease can be characterized using ultrasound-based liver stiffness measurement. To assess its prognostic value, consecutive patients undergoing allogeneic hematopoietic stem cell transplantation were prospectively evaluated in a single-center study. Endpoints included liver event-free survival and all-cause mortality at 1 year. Competing risk and Cox-regression were used for analysis. We evaluated 106 patients (42 female, age 57) and observed 33 life-threatening events (14 died) including 16 liver complications at 100 days. At 1 year, 36 patients had died, 20 with disease relapse. The hazard ratios for liver-related complications at 100 days were 3.2 (95% CI: 1.8–14.6, p = 0.0022) and 4.4 (95% CI: 1.6–11.9, p = 0.0042) for elevated transient elastography (n = 11) and shear-wave velocity (n = 31), respectively. Results were analogous for all-cause mortality at 1 year. Prior stem cell therapy and elevated gamma glutamyltransferase were also associated with outcome. This demonstrates that elastography is a promising and viable tool for risk prediction and should be included in upcoming multi-center trials to establish new means of guiding treatment and prophylaxis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
For original data, please thomas.karlas@medizin.uni-leipzig.de.
Change history
14 March 2019
The original version of this Article was updated to correct some affiliations that were presented incorrectly. David Petroff is in fact the only author at affiliation 2. All other authors listed as being at affiliation 2 (Tina Weiße, Sebastian Beer, Franziska Gnatzy, Joachim Mössner, Michael Tröltzsch, Johannes Wiegand and Volker Keim) are in fact just at affiliation 1. These have now been corrected in the original article.
19 March 2019
In the original article, the affiliations were presented incorrectly. David Petroff is in fact the only author at affiliation 2. All other authors listed as being at affiliation 2 (Tina Weiße, Sebastian Beer, Franziska Gnatzy, Joachim Mössner, Michael Tröltzsch, Johannes Wiegand and Volker Keim) are in fact just at affiliation 1. These have now been corrected in the original article.
References
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. https://doi.org/10.1056/NEJMra052638.
Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015;50:1037–56. https://doi.org/10.1038/bmt.2015.6.
McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450–60. https://doi.org/10.1002/hep.23533.
Strouse C, Zhang Y, Zhang M-J, DiGilio A, Pasquini M, Horowitz MM, et al. Risk score for the development of veno-occlusive disease after allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant. 2018. https://doi.org/10.1016/j.bbmt.2018.06.013.
Dietrich CF, Trenker C, Fontanilla T, Gorg C, Hausmann A, Klein S, et al. New ultrasound techniques challenge the diagnosis of sinusoidal obstruction syndrome. Ultrasound Med Biol. 2018. https://doi.org/10.1016/j.ultrasmedbio.2018.06.002.
EASL-ALEH. Clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64. https://doi.org/10.1016/j.jhep.2015.04.006.
Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall Med. 2017;38:e16–47. https://doi.org/10.1055/s-0043-103952.
Karlas T, Petroff D, Sasso M, Fan J-G, Mi Y-Q, Ledinghen Vde, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30. https://doi.org/10.1016/j.jhep.2016.12.022.
Koch A, Horn A, Dückers H, Yagmur E, Sanson E, Bruensing J, et al. Increased liver stiffness denotes hepatic dysfunction and mortality risk in critically ill non-cirrhotic patients at a medical ICU. Crit Care. 2011;15:R266 https://doi.org/10.1186/cc10543.
Park SH, Lee SS, Sung J-Y, Na K, Kim HJ, Kim SY, et al. Noninvasive assessment of hepatic sinusoidal obstructive syndrome using acoustic radiation force impulse elastography imaging: a proof-of-concept study in rat models. Eur Radiol. 2018;28:2096–106. https://doi.org/10.1007/s00330-017-5179-z.
Karlas T, Weber J, Nehring C, Kronenberger R, Tenckhoff H, Mössner J, et al. Value of liver elastography and abdominal ultrasound for detection of complications of allogeneic hemopoietic SCT. Bone Marrow Transplant. 2014;49:806–11. https://doi.org/10.1038/bmt.2014.61.
Colecchia A, Marasco G, Ravaioli F, Kleinschmidt K, Masetti R, Prete A, et al. Usefulness of liver stiffness measurement in predicting hepatic veno-occlusive disease development in patients who undergo HSCT. Bone Marrow Transplant. 2017;52:494–7. https://doi.org/10.1038/bmt.2016.320.
Reddivalla N, Robinson AL, Reid KJ, Radhi MA, Dalal J, Opfer EK et al. Using liver elastography to diagnose sinusoidal obstruction syndrome in pediatric patients undergoing hematopoetic stem cell transplant. Bone Marrow Transplant. 2018. https://doi.org/10.1038/s41409-017-0064-6.
Trépo E, Romeo S, Zucman-Rossi J, Nahon P. PNPLA3 gene in liver diseases. J Hepatol. 2016;65:399–412. https://doi.org/10.1016/j.jhep.2016.03.011.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. https://doi.org/10.1182/blood-2005-05-2004.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives—a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015;50:781–9. https://doi.org/10.1038/bmt.2015.52.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950–66. https://doi.org/10.1038/ajg.2014.131.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). https://www.eortc.be/services/doc/ctc/.
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. https://doi.org/10.1016/j.jhep.2015.11.004.
Berzigotti A, Castera L. Update on ultrasound imaging of liver fibrosis. J Hepatol. 2013;59:180–2. https://doi.org/10.1016/j.jhep.2012.12.028.
Karlas T, Petroff D, Garnov N, Bohm S, Tenckhoff H, Wittekind C, et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS ONE. 2014;9:e91987 https://doi.org/10.1371/journal.pone.0091987.
Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mossner J, et al. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458–67. https://doi.org/10.3109/00365521.2011.610004.
Karlas T, Lindner F, Troltzsch M, Keim V. Assessment of spleen stiffness using acoustic radiation force impulse imaging (ARFI): definition of examination standards and impact of breathing maneuvers. Ultraschall Med. 2014;35:38–43. https://doi.org/10.1055/s-0033-1356230.
Boursier J, Zarski J-P, Ledinghen V, de, Rousselet M-C, Sturm N, Lebail B, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182–91. https://doi.org/10.1002/hep.25993.
Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–9. https://doi.org/10.1111/j.1365-2893.2011.01537.x.
Rosendahl J, Tonjes A, Schleinitz D, Kovacs P, Wiegand J, Ruffert C, et al. A common variant of PNPLA3 (p.I148M) is not associated with alcoholic chronic pancreatitis. PLoS ONE. 2012;7:e29433 https://doi.org/10.1371/journal.pone.0029433.
R C Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R-project.org/.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. https://doi.org/10.1080/01621459.1999.10474144.
Gray, B. cmprsk: subdistribution analysis of competing risks. 2014. https://CRAN.R-project.org/package=cmprsk.
Bruschi FV, Tardelli M, Claudel T, Trauner M. PNPLA3 expression and its impact on the liver: Current perspectives. Hepat Med. 2017;9:55–66. https://doi.org/10.2147/HMER.S125718.
Mueller S. Does pressure cause liver cirrhosis? The sinusoidal pressure hypothesis. World J Gastroenterol. 2016;22:10482–501.
Nishida M, Kahata K, Hayase E, Shigematsu A, Sato M, Kudo Y et al. Novel ultrasonographic scoring system of sinusoidal obstruction syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018. https://doi.org/10.1016/j.bbmt.2018.05.025.
Salter AI, Pont MJ, Riddell SR. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood. 2018;131:2621–9. https://doi.org/10.1182/blood-2018-01-785840.
Taur Y, Jenq RR, Perales M-A, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–82. https://doi.org/10.1182/blood-2014-02-554725.
Acknowledgements
Tina Weiße was supported by a student’s grant of the Medical Faculty of Leipzig University. We thank Katrin Moritz and Sabrina Fohler (Ultrasound and Endoscopy Unit, Leipzig University Hospital) for organizing the study examinations.
Author information
Authors and Affiliations
Contributions
TK, TW, and VK designed the study, performed the examinations, analyzed the data, and wrote the manuscript. DP analyzed the data and wrote the manuscript. SB, CD, FL, MT, and JW performed the examinations, analyzed the data, and revised the manuscript. DN, GB, and JM treated the patients, reviewed the study examinations, and revised the manuscript. JF performed the genetic analysis and revised the manuscript. G-NF designed the study, performed the examinations, analyzed the data, and wrote the manuscript. All authors had access to the primary clinical trial data.
Corresponding author
Ethics declarations
Conflict of interest
TK, DP, and JW received unrestricted research grants from Echosens, France, not related to the study. TK and VK served as speakers for Siemens Health Care. G-NF received research grants and consulting fees from Jazz Pharmaceuticals, (Palo Alto, USA), Pfizer GmbH (Berlin, Germany), and Novartis Pharma GmbH (Nuremberg, Germany). All the remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Karlas, T., Weiße, T., Petroff, D. et al. Predicting hepatic complications of allogeneic hematopoietic stem cell transplantation using liver stiffness measurement. Bone Marrow Transplant 54, 1738–1746 (2019). https://doi.org/10.1038/s41409-019-0464-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0464-x