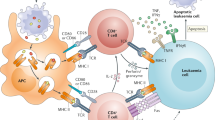
Abstract
Haemopoietic stem cell transplantation is an expanding procedure worldwide but is associated with significant morbidity and mortality. Depletion of resident haemopoietic stem and progenitor cells (HSPC) is required for both autologous and allogeneic haemopoietic stem cell transplantation. Current conditioning protocols utilise chemotherapy or radiation to effectively reduce HSPC but are toxic in both the short and long term. The initial trials to use monoclonal antibodies to target HSPC were limited with marginal efficacy but platforms including antibody drug conjugates and chimeric antigen receptor T cells have made targeted conditioning strategies achievable. In this review we summarise the work developing targeted conditioning that may replace or reduce alkylating agents and total body irradiation. The prospect of conditioning with significantly reduced toxicity will improve outcomes and open transplantation to patients unable to tolerate current conditioning protocols.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
EBMT. EBMT Annual Report. 2017. https://www.ebmt.org/ebmt/documents/ebmt-annual-report-2017.
CIMBTR. CIMBTR Transplant Data. 2018. https://bloodcell.transplant.hrsa.gov/RESEARCH/Transplant_Data/US_Tx_Data/.
Micklem H, Clarke C, Evans E, Ford C. Fate of chromosome-marked mouse bone marrow cells tranfused into normal syngeneic recipients. Transplantation. 1968;6:299–302.
Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–9.
Storb R, Yu C, Barnett T, Wagner JL, Deeg HJ, Nash RA, et al. Stable mixed hematopoietic chimerism in dog leukocyte antigen–identical littermate dogs given lymph node irradiation before and pharmacologic immunosuppression after marrow transplantation. Blood. 1999;94:1131–6.
Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85.
Tjonnfjord G, Steen R, Veiby O, Friedrich W, Egeland T. Evidence for engraftment of donor-type multipotent CD34+ cells in a patient with selective T-lymphocyte reconstitution after bone marrow transplantation for B-SCID. Blood. 1994;84:3584–9.
Müller SM, Kohn T, Schulz AS, Debatin K-M, Friedrich W. Similar pattern of thymic-dependent T-cell reconstitution in infants with severe combined immunodeficiency after human leukocyte antigen (HLA)–identical and HLA-nonidentical stem cell transplantation. Blood. 2000;96:4344–9.
Atilla E, Atilla PA, Demirer T. A review of myeloablative vs reduced intensity/non-myeloablative regimens in allogeneic hematopoietic stem cell transplantations. Balk Med J. 2017;34:1.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–63.
Shouval R, Labopin M, Bondi O, Mishan-Shamay H, Ciceri F, Esteve J, et al. Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: a European Group for Blood and Marrow Transplantation Acute Leukemia Working Party Retrospective Data Mining Study. J Clin Oncol. 2015;33:3144–52.
Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–53.
Bhatia S. Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up. Expert Rev Hematol. 2011;4:437–54.
Brennan B, Shalet SM. Endocrine late effects after bone marrow transplant. Br J Haematol. 2002;118:58–66.
Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–52.
Krishnan A, Bhatia S, Slovak ML, Arber DA, Niland JC, Nademanee A, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–93.
Rizzo JD, Curtis RE, Socié G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–83.
Martin PJ, Counts GW Jr, Appelbaum FR, Lee SJ, Sanders JE, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–6.
Boztug K, Schmidt M, Schwarzer A, Banerjee PP, Díez IA, Dewey RA, et al. Stem-cell gene therapy for the Wiskott–Aldrich syndrome. New Engl J Med. 2010;363:1918–27.
Shaw KL, Garabedian E, Mishra S, Barman P, Davila A, Carbonaro D, et al. Clinical efficacy of gene-modified stem cells in adenosine deaminase–deficient immunodeficiency. J Clin Invest. 2017;127:1689–99.
Kang EM, Malech HL. Gene therapy for chronic granulomatous disease. Methods in enzymology. Vol. 507. Elsevier; USA. 2012. p. 125–54.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–22.
Thompson AA, Walters MC, Kwiatkowski J, Rasko JE, Ribeil J-A, Hongeng S, et al. Gene therapy in patients with transfusion-dependent β-thalassemia. New Engl J Med. 2018;378:1479–93.
Uchida N, Weitzel RP, Platner C, Ballantine J, Bonifacino AC, Price SD, et al. Myeloablative conditioning is required for efficient engraftment of gene-modified cells and prevention of antibody production against transgene products in a rhesus stem cell gene therapy model. Mol Ther. 2015;23:S118–S9.
Shah VO, Civin CI, Loken MR. Flow cytometric analysis of human bone marrow. IV. Differential quantitative expression of T-200 common leukocyte antigen during normal hemopoiesis. J Immunol. 1988;140:1861–7.
Thomas ML, Lefrançois L. Differential expression of the leucocyte-common antigen family. Immunol Today. 1988;9:320–6.
Craig W, Poppema S, Little MT, Dragowska W, Lansdorp PM. CD45 isoform expression on human haemopoietic cells at different stages of development. Br J Haematol. 1994;88:24–30.
Ko S, Deiwick A, Jäger MD, Dinkel A, Rohde F, Fischer R, et al. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nat Med. 1999;5:1292–97.
Dahlke MH, Lauth OS, Jäger MD, Roeseler T, Timrott K, Jackobs S, et al. In vivo depletion of hematopoietic stem cells in the rat by an anti-CD45 (RT7) antibody. Blood. 2002;99:3566–72.
Jäger MD, Vondran FW, Ramackers W, Röseler T, Schlitt HJ, Bektas H, et al. A depleting anti-CD45 monoclonal antibody as isolated conditioning for bone marrow transplantation in the rat. PLoS One. 2016;11:e0154682.
Wulf GG, Luo K-L, Goodell MA, Brenner MK. Anti-CD45–mediated cytoreduction to facilitate allogeneic stem cell transplantation. Blood. 2003;101:2434–9.
Krance RA, Kuehnle I, Rill DR, Mei Z, Pinetta C, Evans W, et al. Hematopoietic and immunomodulatory effects of lytic CD45 monoclonal antibodies in patients with hematologic malignancy. Biol Blood Marrow Transplant. 2003;9:273–81.
Brenner MK, Wulf GG, Rill DR, LUO KL, Goodell MA, Mei Z, et al. Complement‐Fixing CD45 Monoclonal Antibodies to Facilitate Stem Cell Transplantation in Mouse and Man. Ann NY Acad Sci. 2003;996:80–8.
Straathof KC, Rao K, Eyrich M, Hale G, Bird P, Berrie E, et al. Haemopoietic stem-cell transplantation with antibody-based minimal-intensity conditioning: a phase 1/2 study. Lancet. 2009;374:912–20.
McKinney-Freeman SL, Naveiras O, Yates F, Loewer S, Philitas M, Curran M, et al. Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood. 2009;114:268–78.
Shin JY, Hu W, Naramura M, Park CY. High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J Exp Med. 2014;211:217–31.
Russell ES. Hereditary anemias of the mouse: a review for geneticists. Advances in genetics. Vol. 20: Elsevier; USA. 1979. p. 357–459.
Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–25.
Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–20.
Xue X, Pech NK, Shelley WC, Srour EF, Yoder MC, Dinauer MC. Antibody targeting KIT as pretransplantation conditioning in immunocompetent mice. Blood. 2010;116:5419–22.
Derderian SC, Togarrati PP, King C, Moradi PW, Reynaud D, Czechowicz A, et al. In utero depletion of fetal hematopoietic stem cells improves engraftment after neonatal transplantation in mice. Blood. 2014;124:973–80.
Chandrasekaran D, Nakamoto B, Watts KL, Kiem H-P, Papayannopoulou T. Modeling promising nonmyeloablative conditioning regimens in nonhuman primates. Hum Gene Ther. 2014;25:1013–22.
Pang WW, Czechowicz A, Poyser J, Park CY, Weissman IL, Shizuru JA. Anti-Human CD117 antibodies mediate clearance of myelodysplastic syndrome hematopoietic stem cells and facilitate establishment of normal hematopoiesis in transplantation. Biol Blood Marrow Transplant. 2018;24:S230–S1. (Abstract 313)
Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2012;12:58–67.
Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell . 2009;138:271–85.
Willingham SB, Volkmer J-P, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci. 2012;109:6662–7.
Chhabra A, Ring AM, Weiskopf K, Schnorr PJ, Gordon S, Le AC, et al. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci Transl Med. 2016;8:351ra105–351ra105.
Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov. 2017;16:315–37.
Palchaudhuri R, Saez B, Hoggatt J, Schajnovitz A, Sykes DB, Tate TA, et al. Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat Biotechnol. 2016;34:738–45.
Li Z, Czechowicz A, Scheck A, Rossi D, Murphy P. Robust Hematopoietic Mixed Chimerism and Donor-Specific Skin Allograft Tolerance after Non-Genotoxic Anti-CD117 Immunotoxin Conditioning and Donor Bone Marrow Allotransplantation. Am J Transplant. 2018;18:399. (Abstract 398)
Hartigan AJ, Pearse BR, McDonough SM, Proctor JL, Adams HL, McShea MA, et al. A Non-Genotoxic Antibody Drug Conjugate Targeting C-Kit for Hematopoietic Stem Cell Transplant Conditioning. Biol Blood Marrow Transplant. 2018;24:S47–S8. (Abstract 23)
Rivière I, Sadelain M. Chimeric antigen receptors: a cell and gene therapy perspective. Mol Ther. 2017;25:1117–24.
Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. New Engl J Med. 2011;365:725–33.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. New Engl J Med. 2013;368:1509–18.
Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9.
Arai Y, Choi U, Corsino CI, Koontz SM, Tajima M, Sweeney CL, et al. Myeloid conditioning with c-kit-targeted CAR-T cells enables donor stem cell engraftment. Mol Ther. 2018;26:1181–97.
Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor–modified T cells. Blood. 2014;123:2343–54.
Tasian SK, Kenderian SS, Shen F, Ruella M, Shestova O, Kozlowski M, et al. Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood. 2017;129:2395–407.
Gill S, Olson JA, Negrin RS. Natural killer cells in allogeneic transplantation: effect on engraftment, graft-versus-tumor, and graft-versus-host responses. Biol Blood Marrow Transplant. 2009;15:765–76.
Mattsson J, Ringdén O, Storb R. Graft failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:165–70.
Hill GR. Inflammation and bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:139–41.
Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088–103.
Gyurkocza B, Lazarus H, Giralt S. Allogeneic hematopoietic cell transplantation in patients with AML not achieving remission: potentially curative therapy. Bone Marrow Transplant. 2017;52:1083–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abadir, E., Bryant, C., Larsen, S. et al. Targeting the niche: depleting haemopoietic stem cells with targeted therapy. Bone Marrow Transplant 54, 961–968 (2019). https://doi.org/10.1038/s41409-019-0445-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0445-0
This article is cited by
-
Ex vivo editing of human hematopoietic stem cells for erythroid expression of therapeutic proteins
Nature Communications (2020)