Abstract
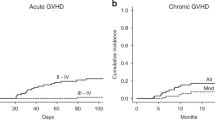
Umbilical cord blood stem cell transplantation (UCBT) is associated with retarded hematopoietic recovery and immune reconstitution and a high infection-related morbidity and mortality, especially after conditioning including anti-thymocyte globulin (ATG). However, data on immune recovery, incidence of infections, and outcome in double UCBT (dUCBT) recipients receiving an ATG-free reduced intensity conditioning (RIC) are lacking. In this study, recovery of lymphocyte subsets, thymopoiesis, and its association with severe infections and clinical outcome was assessed in a group of 55 recipients of a dUCBT ATG-free RIC regimen. T cell recovery was severely protracted in the majority of patients. However, T cell receptor excision circle TREC+ T cells were detectable in 62% of patients at 3 months post-transplantation. A total of 128 common toxicity criteria grade 3−4 infections were observed in the first year post-transplantation. Non-relapse mortality at 12 months post-transplant was 16%, of which 78% infectious mortality. One-year overall survival was 73%. Patients who failed to recover thymopoiesis at 3 months post-transplantation were at a 3.3-fold higher risk of subsequent severe grade 3–4 infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–8.
Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–85.
Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–75.
Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–22.
Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115:1843–9.
Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–60.
Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–81.
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7.
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–9.
Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1.
Somers JA, Braakman E, van der Holt B, Petersen EJ, Marijt EW, Huisman C, et al. Rapid induction of single donor chimerism after double umbilical cord blood transplantation preceded by reduced intensity conditioning: results of the HOVON 106 phase II study. Haematologica. 2014;99:1753–61.
Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–37.
Rufer N, Helg C, Chapuis B, Roosnek E. Human memory T cells: lessons from stem cell transplantation. Trends Immunol. 2001;22:136–41.
Mackall CL, Granger L, Sheard MA, Cepeda R, Gress RE. T-cell regeneration after bone marrow transplantation: differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood. 1993;82:2585–94.
Clave E, Lisini D, Douay C, Giorgiani G, Busson M, Zecca M, et al. Thymic function recovery after unrelated donor cord blood or T-cell depleted HLA-haploidentical stem cell transplantation correlates with leukemia relapse. Front Immunol. 2013;4:54.
Wils EJ, van der Holt B, Broers AE, Posthumus-van Sluijs SJ, Gratama JW, Braakman E, et al. Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1846–54.
Parody R, Martino R, Rovira M, Vazquez L, Vazquez MJ, de la Camara R, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12:734–48.
Safdar A, Rodriguez GH, De Lima MJ, Petropoulos D, Chemaly RF, Worth LL, et al. Infections in 100 cord blood transplantations: spectrum of early and late posttransplant infections in adult and pediatric patients 1996-2005. Medicine (Baltimore). 2007;86:324–33.
Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–51.
Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J, et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis. 2011;13:456–65.
Somers JA, Brand A, van Hensbergen Y, Mulder A, Oudshoorn M, Sintnicolaas K, et al. Double umbilical cord blood transplantation: a study of early engraftment kinetics in leukocyte subsets using HLA-specific monoclonal antibodies. Biol Blood Marrow Transplant. 2013;19:266–73.
Broers AE, van der Holt B, Haze S, Braakman E, Gratama JW, Lowenberg B, et al. A comparison of postengraftment infectious morbidity and mortality after allogeneic partially T cell-depleted peripheral blood progenitor cell transplantation versus T cell-depleted bone marrow transplantation. Exp Hematol. 2005;33:912–9.
Beije N, Versluis J, Kraan J, Gratama JW, Sleijfer S, Cornelissen JJ. Circulating endothelial cell enumeration demonstrates prolonged endothelial damage in recipients of myeloablative allogeneic stem cell transplantation. Haematologica. 2015;100:e246–9.
Danby R, Rocha V. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front Immunol. 2014;5:68.
Niehues T, Rocha V, Filipovich AH, Chan KW, Porcher R, Michel G, et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children—a Eurocord analysis. Br J Haematol. 2001;114:42–8.
Tanaka J, Sugita J, Asanuma S, Arita K, Shono Y, Kikutchi M, et al. Increased number of CD16(+)CD56(dim) NK cells in peripheral blood mononuclear cells after allogeneic cord blood transplantation. Hum Immunol. 2009;70:701–5.
Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002;99:1458–64.
Kanda J, Chiou LW, Szabolcs P, Sempowski GD, Rizzieri DA, Long GD, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1664–76. e1
Brown JA, Stevenson K, Kim HT, Cutler C, Ballen K, McDonough S, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood. 2010;115:4111–9.
Ballen K, Mendizabal AM, Cutler C, Politikos I, Jamieson K, Shpall EJ, et al. Phase II trial of parathyroid hormone after double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2012;18:1851–8.
Simpson D. T-cell depleting antibodies: new hope for induction of allograft tolerance in bone marrow transplantation? BioDrugs. 2003;17:147–54.
Chung B, Barbara-Burnham L, Barsky L, Weinberg K. Radiosensitivity of thymic interleukin-7 production and thymopoiesis after bone marrow transplantation. Blood. 2001;98:1601–6.
Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–9.
Jimenez M, Martinez C, Ercilla G, Carreras E, Urbano-Ispizua A, Aymerich M, et al. Reduced-intensity conditioning regimen preserves thymic function in the early period after hematopoietic stem cell transplantation. Exp Hematol. 2005;33:1240–8.
Chao NJ, Liu CX, Rooney B, Chen BJ, Long GD, Vredenburgh JJ, et al. Nonmyeloablative regimen preserves “niches” allowing for peripheral expansion of donor T-cells. Biol Blood Marrow Transplant. 2002;8:249–56.
Uhlin M, Sairafi D, Berglund S, Thunberg S, Gertow J, Ringden O, et al. Mesenchymal stem cells inhibit thymic reconstitution after allogeneic cord blood transplantation. Stem Cells Dev. 2012;21:1409–17.
Chiesa R, Gilmour K, Qasim W, Adams S, Worth AJ, Zhan H, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naive CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. 2012;156:656–66.
Sauter C, Abboud M, Jia X, Heller G, Gonzales AM, Lubin M, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant. 2011;17:1460–71.
Lindemans CA, Chiesa R, Amrolia PJ, Rao K, Nikolajeva O, de Wildt A, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123:126–32.
Clave E, Busson M, Douay C, Peffault de Latour R, Berrou J, Rabian C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113:6477–84.
Sairafi D, Mattsson J, Uhlin M, Uzunel M. Thymic function after allogeneic stem cell transplantation is dependent on graft source and predictive of long term survival. Clin Immunol. 2012;142:343–50.
Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–66.
John AZ. Prevention of Cytomegalovirus Disease in Hematopoietic Stem Cell Transplantation. Clin Infect Dis. 2002;35(8):999–1004.
Crystal LM, Terry JF, Ronald EG. Harnessing the biology of IL-7 for Therapeutic application. Nat Rev Immunol. 2011;11(5):330–342.
Perales M-A, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, Young JW, Jakubowski AA, Zaidi B, Gallardo H, Liu C, Rasalan T, Wolchok JD, Croughs T, Morre M, Devlin SM, van den Brink MRM. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120(24):4882–4891.
Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood. 2014;124(22):3201–3211.
Dudakov JA, Mertelsmann AM, O’Connor MH, Jenq RR, Velardi E, Young LF, Smith OM, Boyd RL, van den Brink MRM, Hanash AM. Loss of thymic innate lymphoid cells leads to impaired thymopoiesis in experimental graft-versus-host disease. Blood. 2017;130(7):933–942.
Parent AV, Russ HA, Khan IS, LaFlam TN, Metzger TC, Anderson MS, Hebrok M. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell. 2013;13(2):219–229.
Sun X, Xu J, Lu H, Liu W, Miao Z, Sui X, Liu H, Su L, Du W, He Q, Chen F, Shi Y, Deng H. Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo Cell Stem Cell. 2013;13(2):230–236.
Funding
The work described within this manuscript was performed within the framework of the TIPharma project D5-402.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Lucia E. Duinhouwer and Nick Beije contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Duinhouwer, L.E., Beije, N., van der Holt, B. et al. Impaired thymopoiesis predicts for a high risk of severe infections after reduced intensity conditioning without anti-thymocyte globulin in double umbilical cord blood transplantation. Bone Marrow Transplant 53, 673–682 (2018). https://doi.org/10.1038/s41409-018-0103-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0103-y
This article is cited by
-
Dynamics of recent thymic emigrants in pediatric recipients of allogeneic hematopoetic stem cell transplantation
Bone Marrow Transplantation (2022)
-
How to define and measure thymopoiesis after transplantation?
Bone Marrow Transplantation (2018)