Abstract
Study design
Cross-sectional descriptive study.
Objectives
To compare the diffusion tensor imaging (DTI) changes of the sacral cord in people with complete cervical spinal cord injury (SCI) and neurogenic bladder versus people without SCI, and to explore the relationship between sacral cord DTI changes and bladder contractility.
Setting
First Affiliated Hospital of Soochow University, Jiangsu Province, China.
Methods
Forty participants were included: 25 participants with complete cervical SCI and 15 without SCI. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) values were calculated by DTI for ventral horn and intermediate column of sacral cord at S2–S4 level. All participants underwent urodynamic examination. The urodynamic parameters (voiding efficiency (VE), and bladder contractility index (BCI)) and DTI parameters were compared between people with and without SCI. The correlations between DTI values (FA and ADC) and urodynamic parameters were analyzed.
Results
The FA values were significantly lower and the ADC values were significantly higher in the intermediate column and ventral horn at S2–S4 level of the participants with SCI compared with their able-bodied counterparts (p < 0.05). VE and BCI were significantly different between the two groups (p < 0.05). The FA values of intermediate column positively correlated with BCI (r = 0.749, p < 0.05) and the ADC values negatively correlated with BCI (r = −0.471, p < 0.05) in participants with SCI. The DTI values of sacral cord were not correlated with each urodynamic parameter in participants without SCI (p > 0.05).
Conclusions
Complete cervical SCI might lead to microstructural changes of the sacral cord, which might further affect bladder contraction.
Similar content being viewed by others
Introduction
Neurogenic bladder is the most common complication following spinal cord injury (SCI). The regions remote to injury site show permanent anatomic changes and functional reorganization [1]. Some investigators have reported decreases in both the size and numbers of corticospinal neurons as well as changes in synaptic spine density and neuronal morphology following SCI [2]. Many studies have investigated the changes in the brain after SCI in people with imaging [3], but changes in the sacral cord following SCI have not been well studied. The sacral micturition center is located at the S2–S4 level. Parasympathetic efferents from the sacral cord at the S2–S4 level via the pelvic nerves provide excitatory input to the bladder. The external urethral sphincter is regulated through somatic nerves via the pudendal nerve. The detrusor and external urethral sphincter are essential for the function of the lower urethra [4]. Reflex voiding is dependent on the integrity of the sacral micturition center at S2–S4 in people with SCI. Therefore, we need to understand the pathophysiological condition of sacral cord following SCI. Indeed, a better understanding of the pathophysiological condition of sacral cord might provide vital information for the treatment of neurogenic bladder after SCI.
Diffusion tensor imaging (DTI) is a noninvasive MRI method for the quantitative detection of changes in regional neural structures. DTI measures the motion of water molecules in multiple directions and provides indications of tissue integrity. DTI indices such as fractional anisotropy (FA), apparent diffusion coefficient (ADC), axial diffusivity, and radial diffusivity have the potential to quantify biologically relevant diffusion changes in the spinal cord. FA is a parameter of anisotropic strength, ranging from 0 to 1, with values close to 1 indicated relatively strong anisotropy, whereas a value of 0 indicates complete isotropy. FA is a summary measure of microstructural integrity and it is highly sensitive to microstructural changes. A decreased FA value often suggests axonal degeneration or demyelination. ADC values are used to indicate the magnitude of diffusion [3]. DTI assessment of neurological changes rostral to the site of injury had been reported in people with cervical SCI [5]. However, DTI changes of the sacral cord following SCI have not been fully studied. Previous studies reported that DTI values at injury site correlated with motor function and could be used in predicting long-term neurological and functional outcome in people with SCI [6]. Therefore, it might provide further insights into the pathophysiological changes of neurogenic bladder after SCI to reveal the relation between DTI values and bladder function.
The purpose of this study was to investigate DTI changes of the sacral cord in people with complete cervical SCI and neurogenic bladder, comparing with people without SCI, and to explore the DTI value in evaluating the sacral cord microstructure changes and its relation with bladder contractility.
Methods
People with complete cervical SCI with voiding dysfunction were enrolled and designated as the experimental group. All the participants underwent urodynamic studies before enrollment. The inclusion criteria were as follows: (1) the SCI caused by trauma; (2) no concurrent brain injury; (3) clear consciousness and stable vital signs. The exclusion criteria included: (1) sacral cord involved in SCI; (2) more than one incidence of SCI; (3) contraindication for MRI; (4) taking antimuscarinic drugs and skeletal muscle relaxants. Completeness of injury was recorded using the American Spinal Injury Association Classification (ASIA) scale, wherein “A” represents complete injury. The people with SCI included in the experimental group were all with scale “A.” In addition, people without SCI were recruited into the control group, who were from physical examination center, with normal physical examination results. Physical examination items at least contained blood pressure measurement, fasting blood glucose, glycated hemoglobin, lipid analysis, liver and kidney function, blood routine, urine routine, fundus photography, and electrocardiogram. A sufficient detailed interrogation was then followed, to exclude any history disease such as pelvic fractures and prior urinary tract surgery and a history of diabetes. This study was conducted in accordance with the declaration of Helsinki. This study was approved by the Independent Ethics Committee of First Affiliated Hospital of Soochow University. Informed consent was obtained from each participant in the study.
The age and gender of the participants, as well as some clinical characteristics of experimental group (time since injury, neurological level of SCI, ASIA scale, fixation segment, and bladder management) were recorded.
Urodynamic assessment
All participants received urodynamic measurements with the urodynamic instrument (Laborie Delphis B type, Canada). The cystometry and pressure-flow measures were taken with the participants in a seated position. One 8 French (F) triple lumen catheter was transurethrally inserted to detect bladder pressure, and 18F balloon catheter was inserted through the anus to measure abdominal pressure. Sphincter electromyography was performed using surface patch electrodes, which were placed at the 3 and 9 o’clock position around the anus. Detrusor sphincter dyssynergia (DSD) was defined as the presence of involuntary contractions of the external sphincter during detrusor contractions. The following urodynamic parameters were measured: volume voided, postvoid residual, maximum flow rate (Qmax), detrusor pressure at Qmax (PdetQmax), and maximum urethral pressure (MUP). Bladder contractility index (BCI) was calculated using the formula: PdetQmax +5 Qmax. Voiding efficiency (VE) was calculated as: volume voided/(volume voided + postvoid residual) × 100. Participants were asked to avoid abdominal straining during the pressure-flow measurements. All the urodynamic parameters mentioned above were recorded.
MR examination
After emptying the bladder, all the participants underwent MR scans using a 3-Tesla MR scanner (Signa HDxt, GE Healthcare, Milwaukee, WI). A tight girdle was used around the lower abdomen to reduce the respiratory artifacts. Conventional sacral spine MRI (sagittal fast spin echo T1- and T2-weighted sequences, axial fast spin echo T2-weighted sequence) and DTI were obtained. The scan parameters of the DTI sequence were diffusion directions 20, b value 600 mm2/s, repetition time/echo time 6000/80.7 ms, field of view 256 × 256 mm, matrix 128 × 48, slice thickness 2 mm without gap, and number of signal averages (NEX) 3.
Image analysis
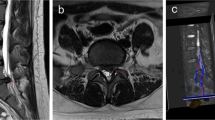
The DTI images were sent to Advanced Workstation (version 4.4). Imaging analyses were performed double-blinded by two attending neuroradiologists. First, DTI images were merged onto the T2-weighted structural images by function tools in the workstation. Then, circular regions of interest (ROI) were manually placed on axial images of the color-coded FA maps at proximately S2–4 sacral cord level, which were respectively and symmetrically placed in bilateral ventral horn and intermediate column of the gray matter of sacral spinal marrow. The ROIs were sequentially named from ROI 1 to ROI 4, with ROI 1 centered in the right ventral horn and ROI 2 in the left side, so were the ROI 3–4 in intermediate column. ROI 1–4 were set carefully to avoid the border areas between the gray matter and white matter of sacral spinal marrow. Finally, the mean FA and ADC values of bilateral ventral horn and intermediate column were respectively calculated and recorded (Fig. 1).
Statistical analysis
The sample size for two independent samples, continuous outcome was calculated according to the formula \(n_i = 2\left( {\frac{{Z\sigma }}{{ES}}} \right)^2\) where ni is the sample size required in each group (i = 1, 2), Z is the value from the standard normal distribution reflecting the confidence level that will be used and E is the desired margin of error. All statistical analyses were performed with standard statistical analysis software (SPSS version 26.0, SPSS). Mann–Whitney U test and two-sample T test were used respectively for nonnormal distributed and normal distributed data, to compare the age, DTI values and urodynamic parameters between the experimental and control groups. Pearson Chi-square test was used to test the difference of the gender between the groups. The Pearson correlation analysis was used to analyze the correlations between DTI values and urodynamic parameters. A p value less than 0.05 was considered statistically significant. The mean value of nonnormal distributed data was recorded as median (IQR) and the mean value of normal distributed data was recorded as mean (SD).
Results
A total of 40 participants (25 people with SCI and 15 without SCI) were enrolled in this study. There were 20 men and 5 women in the experimental group, with a median age 39.0 (12.5) years (mean 95% CI 36.7–47.8 years), and there were 12 men and 3 women in the control group, with a mean age 39.6 (12.7) years (mean 95% CI 32.6–46.6 years). No significant differences were noted with regard to the gender and age (p > 0.05).
In the experimental group, the details of time since injury, neurological level of SCI, fixation segment, and bladder management were showed in the Table 1. All participants with SCI demonstrated neurogenic lower urinary tract dysfunction, among which 4 participants with urinary retention and 21 participants with urinary incontinence. The volumes were less than 100 ml in three participants. Three (12%) participants in the experimental group showed hyporeflexic detrusor, while 20 (80%) presented hyperreflexic detrusor and/or DSD.
The primary outcome of the study was that the people with SCI showed reduced FA value both in intermediate column and ventral horn of sacral cord (p < 0.05) (Table 2), and the FA value of intermediate column was positively correlated with BCI (r = 0.749, p < 0.05) (Fig. 2). The secondary outcomes of the study were as following: (1) the people with SCI showed increased ADC value both in intermediate column and ventral horn of sacral cord (p < 0.05) (Table 2), and the ADC value of intermediate column was negatively correlated with BCI (r = −0.471, p < 0.05) (Fig. 2). (2) The urodynamic parameters (VE and BCI) were significantly different between the experimental group and control group (p < 0.05) (Table 2). (3) Neither FA value nor ADC of ventral horn was statistically associated with MUP in experimental group (respectively r = 0.344, r = −0.154, p > 0.05) (Fig. 3). (4) The DTI values of intermediate column and ventral horn were not correlated with each urodynamic parameter in people without SCI (p > 0.05).
Discussion
In present study, we found that the DTI values of the intermediate column and ventral horn of the sacral cord were significantly different between individuals with and without SCI. The FA value of the intermediate column of the sacral spinal cord showed a positive correlation with BCI, while the ADC value showed a negative correlation with BCI in participants with SCI.
SCI produces a physiologic disconnection with the distal motor targets and disassociation with the sensorimotor areas, which are related to the changes of neural structure and function at the sites remote from the primary injury [5]. Gray and white matter volume is decreased in people with cervical SCI compared with people without SCI [7]. Wrigley et al. reported structural changes in the cortical motor regions and descending motor tracts in people with complete SCI. They speculated that these brain anatomical changes may limit motor recovery following SCI [8]. In another study, Sun et al. found that the value of FA decreased dramatically in the cerebral peduncle of people with cervical ASIA-A/B, and demonstrated both axonal injury and edema/tissue loss occurred in cerebral peduncle of people with cervical ASIA-A/B, but not with cervical ASIA-D and thoracic SCI, as compared with the people without SCI [9]. There were also some reports about the changes of DTI values at the regions near the injury site. The spinal cord area above the injury level showed decreased FA value and it was correlated with poorer motor and sensory function [10]. There were differences of DTI indexes at the injury site and the areas remote from injury site between the injured and control groups [11]. Reduced FA and increased ADC were found in the sacral cord of people with complete cervical SCI in present study, which were consistent with the previous studies [10, 11]. However, Facon et al. found that there was no difference of FA and ADC values in the remote sites away from compression site compared with controls [12]. This inconsistence was mainly due to the different research participants—incomplete SCI and spinal cord compression in Facon’s study verse complete cervical SCI in present study. Mulcahey et al. found significant differences in DTI values between controls and participants with complete SCI while no differences between controls and participants with incomplete SCI at regions above and below injury level [13]. However, none of these studies extended to the microstructural changes of the sacral cord. To the best of our knowledge, this is the first report of decreased FA values and increased ADC values at sacral cord in people with complete cervical SCI. The pathological events that occur at the sites remote to the lesion in people with chronic SCI had been reported [14]. Yokota et al. studied the histological and biological changes in sites remote to the lesion in thoracic SCI mice. They found the number of presynaptic boutons and the expression of neuronal activity makers were significantly lower in lumber motor neurons. The decreased presynaptic input from descending fibers might be responsible for the changes [15].
DTI had been used for the diagnosis and evaluation of various diseases. Decreased FA and the increased ADC generally suggested nerve injury [3]. There was a significant reduction in FA at the whole-cord level in people with acute cervical injury. This finding suggested that DTI might depict the microstructural modifications of the sacral cord (remote from the injury site), even though there was no abnormality detected by conventional MRI [11]. In people with cervical spondylotic myelopathy, FA values of the cervical cord were well correlated with the clinical scores obtained by using the modified Japanese Orthopedic Association scoring system [16]. Moreover, higher FA values were correlated with functional recovery in people with cervical SCI [6].
ADC is affected by cellular size, shape, and integrity and increased in the lesions with edema, demyelination, and axonal loss. FA is a main parameter reflecting the degree of tissue’s anisotropy. Decreased FA indicates fewer axons or less demyelination [3]. In present study, the decreased FA and increased ADC values at the sacral cord suggested that there were microstructural changes of the sacral cord in people with complete cervical SCI and neurogenic bladder. There might be fewer barriers to water molecular movement, indicating a loss of similarly orientated fibers (axons and/or dendrites) and possible cell death in people with complete cervical SCI. In chronic SCI, cellular inflammation and edema were assumed to be less relevant, while axon and myelin loss were thought to be the primary substrate of functional disability [17, 18]. Therefore, our results indicated the pathological changes at sacral cord in people with complete cervical SCI.
Bladder contractility, consisting of contractile strength and contractile duration, was a crucial element for bladder voiding function. Bladder contractility was usually assessed by contractile strength, because it was not well validated to measure contractile duration. Currently, several parameters including Schafer nomogram (pressure/flow), BCI, and Watts Factor may be used to characterize bladder contractility, of which BCI was more commonly used [19]. In this study, reduced BCI was found in the experimental group compared with control group. The intermediate column of gray matter at S2–S4 level was directly pertained to detrusor contraction, because the sacral parasympathetic nervous system, which was originated from the detrusor nucleus located in lamina V–VII of sacral cord, transmitted the major excitatory input to the urinary bladder [4]. After suprasacral SCI, the voluntary control of the lower urinary tract was lost and the spinal reflex control of micturition was presented [20]. It implied that BCI in experimental group was mainly managed by spinal cord micturition center. DTI values at injury site were related with long-term neurological and functional outcome in people with cervical SCI [6]. The present study showed similar results that BCI had a positive correlation with FA and negative correlation with ADC values of intermediate column at S2–S4 level, suggesting that the pathological changes occurring in sacral cord (S2–S4, spinal cord micturition center) might be the cause of reduced BCI in people with complete cervical SCI. Six participants in the experimental group showed normal BCI (>100), while they showed poor empty (postvoid residual volume more than 100 ml) due to DSD. According to the BCI formula, BCI was partially depended on Qmax. Since the abdominal strain during voiding would increase BCI artificially, it was excluded by asking participants to relax the abdominal muscles during pressure-flow study. Contractility was a physiologic term used to describe muscle performance. Currently used parameters of bladder contractility were derived from pressure-flow studies in people without SCI. Since each parameter was not the direct index to measure detrusor contractility and has its certain limitations, there was no universally accepted method to assess bladder contractility so far [21]. Although BCI was used to assess detrusor contractility in this study as in some previous studies [22, 23], further assessments were still needed in future study design. It was possible that BCI might partially describe bladder voiding function in people with SCI. In order to gain a more accurate assessment of bladder voiding function, VE was also selected and analyzed in this study. Given the anatomical differences of genitourinary system, there was such a view that BCI should be used with caution in women as it might be highly influenced by reduced outflow resistance [24]. Hence, it was advisable to explore and define a urodynamic-based parameter without gender-related difference to reflect physiological bladder contractility in people with SCI.
VE was an indicator of bladder voiding function. VE was affected by coordinated actions between the detrusor and the urethral sphincter, which was controlled by the central nervous and peripheral and autonomic nervous system. Complete suprasacral injuries disrupt the neuronal pathways between the brain and sacral micturition center. The VE was 46% and showed no significant correlation with DTI values in the experimental group. This might be due to DSD, which results in a large residual volume of urine. Contrary to the findings of the experimental group, no obvious correlation was found between DTI values in sacral cord and BCI in control group. This suggests that effects of sacral micturition center on micturition may be different in people with SCI and without SCI.
The external urethral sphincter played a major role in MUP. Although the external urethral sphincter was innervated by somatic nervous system, which was arising from the pudendal (Onuf’s) nucleus located in lamina IX of sacral cord [4], our results showed that there was no obvious correlation between DTI values of the ventral horn in the sacral cord and MUP in people with complete cervical SCI. This result might be due to the following two reasons. First, the urethral pressure measured in this study was static, but actually the urethral pressure varied throughout voiding in people with neurogenic bladder and these changes of urethral pressure were more obvious in people with SCI with detrusor external sphincter dyssynergia [25]. Second, prostate was an important factor affecting the urinary pressure of male participants [26] and 80% of participants (20/25) in this study were male. Therefore, the MUP may not fully reflect the function of the external urinary sphincter in people with SCI.
There was a general correlation between the neurological level of injury and bladder behavior in people with SCI. Theoretically, participants with high thoracic or cervical SCI normally have uninhibited detrusors contraction. Eighty percent of people with cervical SCI showed hyperreflexic detrusor and/or DSD in this study, which was similar to previous research [27]. But some of them show atypical signs and symptoms, such as urinary retention, hyporeflexic, and areflexic bladder [28]. Tosi’s study reported that five participants (14%) high thoracic or cervical SCI showed bladder hyporeflexia [29]. They found that the whole-cord distal to injury site became atrophic in two cases, and the sacral cord showed multiple cystic cavities in other cases with MRI. In our study, no abnormality was found in the sacral cord except for the changes of DTI values with MRI. It was suggested that multiple factors, especially the different structural changes of spinal cord might be responsible for the atypical symptoms.
The main limitation of this study was the relatively small sample size of the two groups, which may lead to less reliable results. A higher number of people with SCI should be gathered for a more robust data set in the future. Furthermore, we focused on the people with complete SCI. However, the differences of DTI values between individuals with complete and incomplete SCI were not included. In addition, there are different types of neurogenic bladder, which may affect the DTI value of the sacral cord in people with SCI. A further study needs to be designed on DTI changes of the sacral cord in individuals with SCI and different types of neurogenic bladder.
In conclusion, there are DTI changes of the sacral cord in people with complete cervical SCI and neurogenic bladder, and these changes significantly correlate with detrusor contraction. It suggests that DTI might be a noninvasive and promising method to reflect the severity of the sacral microstructural change, and to explore the etiology of neurogenic lower urinary tract dysfunction after SCI, but further studies are needed to confirm this hypothesis.
Data availability
The datasets analyzed during the current study are available from corresponding author on reasonable request.
References
Cadotte DW, Fehlings MG. Spinal cord injury: visualizing plasticity and repair in the injured CNS. Nat Rev Neurol. 2013;9:546–7.
Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol. 2006;198:401–15.
Kanamoto H, Norimoto M, Eguchi Y, Oikawa Y, Orita S, Inage K, et al. Evaluating spinal canal lesions using apparent diffusion coefficient maps with diffusion-weighted imaging. Asian Spine J. 2020. https://doi.org/10.31616/asj.2019.0266.
de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol. 2015;5:327–96.
Guleria S, Gupta RK, Saksena S, Chandra A, Srivastava RN, Husain M, et al. Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. J Neurosci Res. 2008;86:2271–80.
Shanmuganathan K, Zhuo J, Chen HH, Aarabi B, Adams J, Miller C, et al. Diffusion tensor imaging parameter obtained during acute blunt cervical spinal cord injury in predicting long-term outcome. J Neurotrauma. 2017;34:2964–71.
Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, et al. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;134:1610–22.
Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, et al. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–32.
Sun P, Murphy RK, Gamble P, George A, Song SK, Ray WZ. Diffusion assessment of cortical changes, induced by traumatic spinal cord injury. Brain Sci. 2017;7:21–33.
Koskinen E, Brander A, Hakulinen U, Luoto T, Helminen M, Ylinen A, et al. Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma. 2013;30:1587–95.
Cheran S, Shanmuganathan K, Zhuo J, Mirvis SE, Aarabi B, Alexander MT, et al. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma. 2011;28:1881–92.
Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. Am J Neuroradiol. 2005;26:1587–94.
Mulcahey MJ, Samdani AF, Gaughan JP, Barakat N, Faro S, Shah P, et al. Diagnostic accuracy of diffusion tensor imaging for pediatric cervical spinal cord injury. Spinal Cord. 2013;51:532–7.
Min KJ, Jeong HK, Kim B, Hwang DH, Shin HY, Nguyen AT, et al. Spatial and temporal correlation in progressive degeneration of neurons and astrocytes in contusion-induced spinal cord injury. J Neuroinflammation. 2012;9:100.
Yokota K, Kubota K, Kobayakawa K, Saito T, Hara M, Kijima K, et al. Pathological changes of distal motor neurons after complete spinal cord injury. Mol Brain. 2019;12:4.
Ellingson BM, Salamon N, Grinstead JW, Holly LT. Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J. 2014;14:2589–97.
Kitzman P. Alterations in axial motoneuron morphology in the spinal cord injured spastic cat. Exp Neurol. 2005;192:100–8.
Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–69.
Aldamanhori R, Chapple CR. Underactive bladder, detrusor underactivity, definition, symptoms, epidemiology, etiopathogenesis, and risk factors. Curr Opin Urol. 2017;27:293–9.
Tai C, Roppolo JR, de Groat WC. Spinal reflex control of micturition after spinal cord injury. Restor Neurol Neurosci. 2006;24:69–78.
Ahmed A, Farhan B, Vernez S, Ghoniem GM. The challenges in the diagnosis of detrusor underactivity in clinical practice: a mini-review. Arab J Urol. 2016;14:223–7.
Lombardi G, Musco S, Celso M, Ierardi A, Nelli F, Del Corso F, et al. Intravesical electrostimulation versus sacral neuromodulation for incomplete spinal cord patients suffering from neurogenic non-obstructive urinary retention. Spinal Cord. 2013;51:571–8.
Chen SF, Lee CL, Kuo HC. Change of detrusor contractility in patients with and without bladder outlet obstruction at ten or more years of follow-up. Sci Rep. 2019;9:18887.
Smith PP, Valentini F, Mytilekas KV, Apostolidis A, Rademakers K, Cardozo L, et al. Can we improve our diagnosis of impaired detrusor contractility in women? An ICI-RS 2019 proposal. Neurourol Urodyn. 2019. https://doi.org/10.1002/nau.24260.
Vírseda M1, Salinas J, López A, Esteban M. Usefulness of Dynamic Urethral Resistance Relation (DURR) measurement for differential diagnosis between static and dynamic urinary obstruction in male spinal cord injury patients. Neurourol Urodyn. 2012;31:549–55.
Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–40.
Weld KJ, Dmochowski RR. Association of level of injury and bladder behavior in patients with post-traumatic spinal cord injury. Urology. 2000;55:490–4.
Berić A, Dimitrijević MR, Light JK. A clinical syndrome of rostral and caudal spinal injury: neurological, neurophysiological and urodynamic evidence for occult sacral lesion. J Neurol Neurosurg Psychiatry. 1987;50:600–6.
Tosi L, Righetti C, Terrini G, Zanette G. Atypical syndromes caudal to the injury site in patients following spinal cord injury. A clinical, neurophysiological and MRI study. Paraplegia. 1993;31:751–6.
Acknowledgements
We would specially like to thank Dr Weixin Yang, Dr Li Li, Dr Shaofeng Zhao, Dr Qingmei Chen, Dr Kai Liu, Dr Haibo Wang, Dr Boye Ni, Dr Hongbing Zhang for their precious help and support.
Funding
The present study was supported by the National Natural Science Foundation of China (Grant no. 81971573) and the Project of Invigorating Health Care through Science, Technology and Education, Jiangsu Provincial Medical Youth Talent (grant no. QNRC2016709).
Author information
Authors and Affiliations
Contributions
HZ was responsible for designing the study, collecting and analyzing data, revising the paper. HD was responsible for designing the study, MR examination, collecting and analyzing data, writing the paper. DZ and LZ were responsible for urodynamic assessment and collecting data. CL, YZ, and PC contributed to screening potential participants and collecting data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, H., Zhu, H., Zhang, D. et al. The correlation between diffusion tensor imaging of the sacral cord and bladder contractility in people with tetraplegia. Spinal Cord 58, 1255–1262 (2020). https://doi.org/10.1038/s41393-020-0484-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-0484-9