Abstract
Study design
Case-control study.
Objectives
The objective of this study was to provide some useful information concerning the incidence, clinical features, and risk factors for symptomatic postoperative spinal epidural hematoma (SPSEH) in an isolated cohort of patients undergoing spine tumor surgery.
Setting
Hospital in Shanghai, China.
Methods
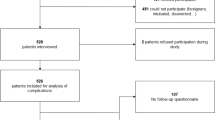
We retrospectively reviewed all patients who underwent surgery for spine tumors between August 2012 and August 2017, and conducted a case-control study involving 16 patients who received evacuation surgery due to SPSEH after spine tumor surgery and 48 controls without SPSEH. Case and control subjects were matched at 1:3 by pathological diagnosis, tumor size (±1 cm), resection mode, surgical approach, and the operation team. Data of SPSEH subjects along with 48 matched controls were further obtained from a detailed review of the medical records. Univariate and multivariate analyses were conducted to identify the risk factors for developing SPSEH.
Results
SPSEH evacuation surgery was performed after 16 of 5421 (0.30%) spine tumor surgeries. Angiogenic tumors were the most susceptible tumors developing SPSEH. Very large hematomas, continuous blood loss, and delayed hematomas were characteristic clinical presentations for SPSEH after spine tumor surgery. Multiple logistic regression analysis suggested that patients suffering from at least one medical comorbidity and patients with Frankel grade of A-C had a significantly higher risk of developing SPSEH.
Conclusions
The incidence of SPSEH after spine tumor surgery requiring surgical evacuation was 0.30%. Medical comorbidity and Frankel grade were identified as independent risk factors for SPSEH development.
Similar content being viewed by others
Introduction
Symptomatic postoperative spinal epidural hematoma (SPSEH) is considered to be one of the most urgent and hazardous complications after spine surgery. The incidence of asymptomatic postoperative spinal epidural hematoma identified by magnetic resonance imaging (MRI) was reportedly 33–58% [1, 2], while 0–0.7% patients received surgical evacuation of SPSEH [3,4,5,6,7,8]. Although rare, SPSEH could potentially leave patients with the devastating neurological consequence [9]. Identifying risk factors of SPSEH could help surgeons make an early diagnosis and give timely treatments for this serious complication.
Recent studies have revealed numerous risk factors for SPSEH development, including hypertension, poor postoperative drainage, spinal puncture, trauma, pregnancy, nonsteroidal anti-inflammatory drug (NSAID) use, higher body mass index, intraoperative use of gelfoam for dura coverage, alcohol consumption, previous spinal surgery, multilevel procedures, older than 60 years of age, Rh-positive blood type, international normalized ratio >2.0, and preoperative coagulopathy [1, 3, 4, 6, 8, 10,11,12,13,14]. However, the incidence, characteristic clinical manifestations, and risk factors of SPSEH after spine tumor surgery still remain unclear.
Comparing with routine spine surgery, spine tumor surgery is characterized with larger surgical trauma, more blood loss, and severer postoperative complications [15,16,17]. With the improvement of surgical technique, more and more spine tumor surgeries are performed, thus their postoperative complications, SPSEH included, will be encountered more frequently in clinical practice. Therefore, we feel that a focused investigation of SPSEH in patients undergoing spine tumor surgery is warranted. In this study, we retrospectively reviewed all spine tumor surgeries performed in our center in recent five years, to determine the incidence of SPSEH after spine tumor surgery and identify potential risk factors for its development.
Methods
Among the 5421 spine tumor surgeries (vertebral, extradural, intradural extramedullary, and partially intramedullary tumors) performed in our spine tumor center between August 2012 and August 2017, 16 patients who received evacuation surgery due to SPSEH were included in the present study. This study was approved by the hospital ethics committee, and informed consent was obtained from each patient.
The administration of anti-coagulant and antiplatelet drugs was discontinued in accordance to the drug medical guidelines before surgery, and the results of coagulation function tests (PT, APTT, INR, and fibrinogen) of all operative patients were in the normal range. The surgical protocol consisted of decompression of the spinal cord, tumor excision, reconstruction and stabilization of the spine. Suction drainage was normally implanted intraoperatively. After the operation, the drain was removed when the amount of bleeding was <50 mL per day. Anticoagulation therapy was resumed 24 h after the removal of the suction drain. The diagnosis of SPSEH was made based on the manifestation of unusually aggravated back or lower limb pain, motor deterioration, bladder disturbances, and the intraoperative finding (evacuation surgery) that hematoma severely compressed the dura [18].
To investigate the risk factors for SPSEH, another 48 patients were selected as a control group. Controls were selected among patients who received spine tumor surgery in our center but without developing SPSEH. Case and control subjects were matched at 1:3 by pathological diagnosis, tumor size (±1 cm), resection mode, surgical approach, and the operation team. Among all patients who met the eligibility and matching criteria, only those who had the shortest three time intervals with the SPSEH one in terms of surgery date were recruited into the control group. A detailed review of the medical records was carried out for all 64 patients by two independent reviewers. Possible risk factors were categorized into three groups: patient-, tumor-, and treatment-related factors. Patient-related factors were blood pressure, tobacco use, alcohol consumption, medical comorbidity, neurological status (Frankel grade), pre- and post-operative hemoglobin and platelet level. Tumor-related factors included tumor site, radiotherapy, and chemotherapy. Treatment-related factors contained preoperative NSAID use, preoperative anticoagulation, previous spinal surgery, preoperative embolization, number of levels of spinal decompression, intraoperative blood loss, red blood cells transfusion, plasma transfusion, operation time, and postoperative deep vein thrombosis (DVT) chemoprophylaxis.
Statistical analyses were performed using the SPSS Statistics, version 22.0 (IBM corp., New York, USA). All the above-mentioned patient-, tumor-, and treatment-related factors were entered into univariate analyses, in which continuous variables were compared by Student’s t test, and categorical variables were compared using χ2 test. All variables significant at p < 0.05 in the univariate analyses were subjected to multivariate analysis using multiple logistic regression analysis. Adjusted odds ratios and 95% confidence intervals were calculated. Variables in the multivariate analysis with p values of <0.05 were recognized as the independent significant risk factors for SPSEH.
Results
Incidence of SPSEH
Of 5421 patients receiving spine tumor surgery in our spine tumor center from 2012 to 2017, 16 patients developed SPSEH and required reoperation for hematoma evacuation. The incidence rate was ~0.30%.
Clinical features of SPSEH
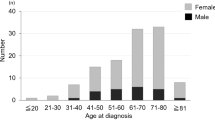
The general data of SPSEH patients is listed in Table 1. Angiogenic tumors, including hemangioma, aggressive hemangioma, hemangiosarcoma and epithelioid hemangioendothelioma, were the most common tumors developing SPSEH (5 cases, 31%). The mean intraoperative blood loss was 2619 mL (range, 600–7000) for all SPSEH patients. 13 patients (81%) received a blood transfusion with the mean red blood cells and plasma transfusion of 1800 mL (range, 400–4400) and 950 mL (range 200–2200), respectively.
Besides common signs of nerve or spinal cord such as intractable pain, loss of muscle power, and saddle anesthesia, SPSEH patients with spine tumor surgery also showed characteristic symptoms. Patients with paravertebral or sacral huge hematoma (Case 7, 9, and 13) manifested as persistent internal bleeding, hemoglobin decreasing, and even hemorrhagic shock. The time interval between primary spine tumor surgeries and the onset of their symptoms ranged from 3 h to 1 month (median, 16 h). In addition, symptoms appeared more than 48 h after surgery in six cases (38%). All hematoma evacuation surgeries were performed within 8 h after presenting symptoms of incomplete paralysis. At discharge, seven patients (44%) got complete neurological function recovery; six (38%) partial recovery, and three (19%) no improvement.
Risk factors for SPSEH
There was no statistically significant difference between the two groups with regard to baseline characteristics (Table 2). The results of univariate analyses to identify the potential risk factors associated with SPSEH are shown in Table 3. Patients in the SPSEH group had more medical comorbidities (P = 0.013), poorer neurological status (Frankel grade, P < 0.001), more intraoperative blood loss (P = 0.040), and lower postoperative hemoglobin level (P = 0.028) than patients in the control group. There were no significant differences in other patient-, tumor-, and treatment-related factors.
Four potential risk factors selected from univariate analyses (medical comorbidities, Frankel grade, postoperative hemoglobin, and intraoperative blood loss) were subjected to multivariate analysis. Table 4 shows the results of multiple logistic regression analysis to determine independent risk factors for SPSEH. The likelihood of developing SPSEH was significantly increased in patients with at least one medical comorbidity (P = 0.023, OR = 5.33, 95%CI: 1.26–22.49). Patients with A-C of the Frankel grade had a significantly higher risk of developing SPSEH (P = 0.013, OR = 6.17, 95%CI: 1.47–25.95). The result of multivariate analysis also showed that postoperative hemoglobin level (P = 0. 280) and intraoperative blood loss (P = 0. 159) were not independent risk factors for SPSEH.
Discussion
Spine tumor surgery is a complicated and challenging work with a high risk of postoperative complications, among which SPSEH is not to be ignored. The incidence of SPSEH seems to fluctuate with different operating procedures. Kao et al. reported that the incidence of SPSEH in patients who underwent lumbar decompression procedure was 0.16% [12]. For patients who received microscopic lumbar decompression surgery, the reoperation rate due to SPSEH raised to 0.6% [10]. Goldstein et al. reported that the incidence of SPSEH after posterior cervical surgery was higher and up to 1.5% [11]. In general, it was reported that 0-0.7% patients after all kinds of spine surgeries suffered from SPSEH and required reoperation [3,4,5,6,7,8]. To our knowledge, our study is the first to examine the incidence of SPSEH in an isolated cohort of patients undergoing spine tumor surgery. Our results showed that SPSEH occurred at an overall rate of 0.30%, which fell within the range from 0 to 0.7%, as previously reported. Aside from the discrepancy caused by differences in the study populations and other uncontrollable bias, we fail to find the obvious difference of SPSEH incidence between spine tumor surgery and other kinds of spine surgeries.
Initially, the common symptom of SPSEH is wound and innervated area pain. As hematoma develops, it would cause spinal canal compromise and nerve root compression, leading to neurological deficits, such as limbs weakness, sensory disturbance, and detrusor dysfunction [12, 17, 19]. Particularly, based on our results, very large hematoma and continuous blood loss was not uncommon after spine tumor surgery. Furthermore, it was reported that symptoms of SPSEH often occur within 24 h after initial spine surgery (rarely more than three days), and the average interval from symptom onset to the maximum deficit was around 12 h [5, 19, 20]. In our series, symptoms of SPSEH were observed more than 48 h after surgery in six cases (38%), with the longest time interval of one month. These delayed hematomas might result from extensive resection range of spine tumor surgery leaving more space for spine cord and nerve root. Therefore, patients sometimes experienced an asymptomatic period before the onset of neurologic symptoms.
Previous study suggested that internal vertebral venous plexus played an important role in the etiology of the spontaneous spinal epidural hematoma [21]. In our study, 5 of 16 patients (31%) were diagnosed with angiogenic tumors. The highly vascular nature of this kind of tumor increases the risk of rupture of vascular plexus, thus placing patients at an increased risk of hematoma formation. On the other hand, angiogenic tumors are usually accompanied by high blood loss. Awad et al. regarded blood loss >1 L and hemoglobin <10 g/dL as risks factors for SPSEH [6]. Similarly, intraoperative blood loss and postoperative hemoglobin level were also showed statistical differences between two groups in our univariate analysis. Stated thus, we infer that angiogenic tumor might be most susceptible to SPSEH.
It is generally accepted that patients who lose the ambulatory ability and lie in bed are related with thrombosis rather than hemorrhage, and the incidence of deep venous thromboembolism within 3 months of paralytic spinal cord injury was reportedly 38% [22]. Nevertheless, based on the data presented in our study, we interestingly found that patients with Frankel grade of A-C were an independent risk factor for SPSEH. Further researches are expected to clarify the mechanism of the poor neurological function in the formation of SPSEH.
Previous investigations have demonstrated that liver diseases, myeloproliferative disorders, kidney diseases, autoimmune diseases, etc. were risk factors for SPSEH [6, 8, 23, 24]. In our study, patients with at least one medical comorbidity were found to be associated with a higher risk of SPSEH independently. Due to the limited number of patients with various comorbidities, we did not investigate the association between SPSEH and specific diseases. Charlson Comorbidity Index (CCI) was recommended as a weighted method to classify patients’ comorbidity, and Goldstein et al. also drew a similar conclusion that increased CCI was a significant predictor of SPSEH after posterior cervical surgery [11, 25].
Postoperative DVT chemoprophylaxis (low-molecular-weight heparin 5000 IU daily) was generally used for anticoagulation therapy for patients who were at the high risk of DVT (evaluated by Caprini risk score). Considering the risk of bleeding after the tumor surgery, we started the anticoagulation therapy 24 h after the removal of the suction drain. We further analyzed the correlation of postoperative DVT chemoprophylaxis with the development of hematoma, but we failed to find postoperative DVT chemoprophylaxis as a predisposing risk factor. Actually, only one case in the SPSEH group received postoperative DVT chemoprophylaxis, while most cases developed SPSEH and underwent the evacuation surgery before removal of the suction drain and starting the anticoagulation therapy.
The degree of resection with the development of postoperative hematomas is an interesting issue, with the assumption that subtotal resection with residual tumor might have much more risk of postoperative hematoma. However, based on our study design, resection mode (total en bloc resection vs total piecemeal resection vs subtotal resection) was matched between case and control groups. As a result, the degree of resection was not analyzed in the present study.
Preoperative embolization is a common practice nowadays for vascular tumors to reduce intraoperative blood loss and might help reduce the risk of postoperative hematomas. The result of our study showed that the proportion of patients receiving preoperative embolization was higher in the control group, but the difference was not significant. Other factors, such as high blood pressure, preoperative NSAID or anticoagulation use, previous spinal surgery, and multiple levels of spinal decompression were regarded as risk factors for SPSEH by several studies [1, 3, 4, 6, 8, 10, 11]. However, there were no significant differences in our series. The role of those factors in developing SPSEH after spine tumor surgery would be the next research focus.
Our study has several limitations. Due to the retrospective nature, limited sample size, and the heterogeneity of spine tumors, bias and confounders are hard to avoid. Despite these limitations, our study provides a wealth of information regarding SPSEH in an isolated cohort of patients undergoing spine tumor surgery.
In conclusion, the incidence of SPSEH requiring surgical evacuation in 5421 spine tumor surgeries was 0.30%. The angiogenic tumor might be most susceptible to SPSEH. Very large hematomas, continuous blood loss, and delayed hematomas could be regarded as clinical features of SPSEH after spine tumor surgery. Multiple logistic regression analysis showed that suffering from medical comorbidity and Frankel grade of A-C were independent risk factors for SPSEH.
Data archiving
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sokolowski MJ, Garvey TA, Perl J 2nd, Sokolowski MS, Cho W, Mehbod AA, et al. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine . 2008;33:108–13.
Ikuta K, Tono O, Tanaka T, Arima J, Nakano S, Sasaki K, et al. Evaluation of postoperative spinal epidural hematoma after microendoscopic posterior decompression for lumbar spinal stenosis: a clinical and magnetic resonance imaging study. J Neurosurg Spine. 2006;5:404–9.
Yamada K, Abe Y, Satoh S, Yanagibashi Y, Hyakumachi T, Masuda T. Large Increase in Blood Pressure After Extubation and High Body Mass Index Elevate the Risk of Spinal Epidural Hematoma After Spinal Surgery. Spine . 2015;40:1046–52.
Amiri AR, Fouyas IP, Cro S, Casey AT. Postoperative spinal epidural hematoma (SEH): incidence, risk factors, onset, and management. Spine J. 2013;13:134–40.
Aono H, Ohwada T, Hosono N, Tobimatsu H, Ariga K, Fuji T, et al. Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. J Neurosurg Spine. 2011;15:202–5.
Awad JN, Kebaish KM, Donigan J, Cohen DB, Kostuik JP. Analysis of the risk factors for the development of post-operative spinal epidural haematoma. J Bone Jt Surg Br. 2005;87:1248–52.
Glotzbecker MP, Bono CM, Wood KB, Harris MB. Postoperative spinal epidural hematoma: a systematic review. Spine. 2010;35:E413–20.
Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine. 2002;27:1670–3.
Giugno A, Basile L, Maugeri R, Iacopino DG. Emergency surgery in a patient with large spontaneous spinal epidural hematoma determining excellent neurological recovery: review of the literature. Spinal Cord. 2014;52(Suppl 3):S22–4.
Fujiwara Y, Manabe H, Izumi B, Harada T, Nakanishi K, Tanaka N, et al. The impact of hypertension on the occurrence of postoperative spinal epidural hematoma following single level microscopic posterior lumbar decompression surgery in a single institute. Eur Spine J. 2017;26:2606–15.
Goldstein CL, Bains I, Hurlbert RJ. Symptomatic spinal epidural hematoma after posterior cervical surgery: incidence and risk factors. Spine J. 2015;15:1179–87.
Kao FC, Tsai TT, Chen LH, Lai PL, Fu TS, Niu CC, et al. Symptomatic epidural hematoma after lumbar decompression surgery. Eur Spine J. 2015;24:348–57.
Domenicucci M, Mancarella C, Santoro G, Dugoni DE, Ramieri A, Arezzo MF, et al. Spinal epidural hematomas: personal experience and literature review of more than 1000 cases. J Neurosurg Spine. 2017;27:198–208.
Lillemae K, Jarvio J, Silvasti-Lundell MK, Antinheimo J, Hernesniemi J, Niemi TT. Incidence of postoperative hematomas requiring surgical treatment in neurosurgery: a retrospective observational study. World Neurosurg. 2017;108:491–7.
Boriani S, Gasbarrini A, Bandiera S, Ghermandi R, Lador R. Predictors for surgical complications of en bloc resections in the spine: review of 220 cases treated by the same team. Eur Spine J. 2016;25:3932–41.
Luzzati AD, Shah S, Gagliano F, Perrucchini G, Scotto G, Alloisio M. Multilevel en bloc spondylectomy for tumors of the thoracic and lumbar spine is challenging but rewarding. Clin Orthop Relat Res. 2015;473:858–67.
Yang W, Jiang L, Liu X, Wei F, Yu M, Wu F, et al. Surgical complications of extraspinal tumors in the cervical spine: a report of 110 cases and literature review. Eur Spine J. 2018;27:882–90.
Shin HK, Choi I, Roh SW, Rhim SC, Jeon SR. Relevance of postoperative magnetic resonance images in evaluating epidural hematoma after thoracic fixation surgery. World Neurosurg. 2017;107:803–8.
Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg. 1995;83:1–7.
Uribe J, Moza K, Jimenez O, Green B, Levi AD. Delayed postoperative spinal epidural hematomas. Spine J. 2003;3:125–9.
Groen RJ, Ponssen H. The spontaneous spinal epidural hematoma. A study of the etiology. J Neurol Sci. 1990;98:121–38.
Anderson FA Jr., Spencer FA. Risk factors for venous thromboembolism. Circulation . 2003;107(23Suppl 1):I9–16.
Laglia AG, Eisenberg RL, Weinstein PR, Mani RL. Spinal epidural hematoma after lumbar puncture in liver disease. Ann Intern Med. 1978;88:515–6.
Shahlaie K, Fox A, Butani L, Boggan JE. Spontaneous epidural hemorrhage in chronic renal failure. A case report and review. Pedia Nephrol. 2004;19:1168–72.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Author information
Authors and Affiliations
Contributions
Conception and design: T Liu, Xiao, Gao; Acquisition of data: Gao, Li, Cao; Analysis and interpretation of data: Gao, Li, Cao; Drafting the article: Gao.; Statistical analysis: Zhao, Y Liu; Reviewed submitted version of manuscript: Yang; Administrative/technical/material support: Dong; Revising the article: Wan; Study supervision: Xiao, T Liu, Wan.
Corresponding authors
Ethics declarations
Ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, X., Li, L., Cao, J. et al. Symptomatic postoperative spinal epidural hematoma after spine tumor surgery: Incidence, clinical features, and risk factors. Spinal Cord 57, 708–713 (2019). https://doi.org/10.1038/s41393-019-0281-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-019-0281-5
This article is cited by
-
Efficacy and safety of erythropoietin in isolated spinal metastasis patients with total en bloc spondylectomy surgery: a case–control study
European Spine Journal (2023)
-
Incidence of postoperative symptomatic spinal epidural hematoma requiring surgical evacuation: a systematic review and meta-analysis
European Spine Journal (2022)
-
Complications of spine surgery for metastasis
European Journal of Orthopaedic Surgery & Traumatology (2020)