Abstract
Objectives
To assess the efficacy of high-intensity interval training (HIIT) for improving cardiorespiratory fitness (CRF) in patients awaiting resection for urological malignancy within four weeks.
Subjects/patients and methods
A randomised control trial of consecutive patients aged (>65 years) scheduled for major urological surgery in a large secondary referral centre in a UK hospital. The primary outcome is change in anaerobic threshold (VO2AT) following HIIT vs. standard care.
Results
Forty patients were recruited (mean age 72 years, male (39): female (1)) with 34 completing the protocol. Intention to treat analysis showed significant improvements in anaerobic threshold (VO2AT; mean difference (MD) 2.26 ml/kg/min (95% CI 1.25–3.26)) following HIIT. Blood pressure (BP) also significantly reduced in following: HIIT (SBP: −8.2 mmHg (95% CI −16.09 to −0.29) and DBP: −6.47 mmHg (95% CI −12.56 to −0.38)). No reportable adverse safety events occurred during HIIT and all participants achieved >85% predicted maximum heart rate during sessions, with protocol adherence of 84%.
Conclusions
HIIT can improve CRF and cardiovascular health, representing clinically meaningful and achievable pre-operative improvements. Larger randomised trials are required to investigate the efficacy of prehabilitation HIIT upon different cancer types, post-operative complications, socio-economic impact and long-term survival.
Similar content being viewed by others
Introduction
Urological malignancy is common, with over 69,000 new diagnoses for prostate, kidney and bladder cancer in 2015 in the United Kingdom (UK) alone [1]. Surgery for these cancers is associated with high complication rates (cystectomy 56%, nephrectomy 21%, prostatectomy 19%) [2] and survivors commonly experience fatigue, reduced physical ability and reduced quality of life [3]. To exemplify this, only 30% of survivors have returned to baseline levels of physical function at 3 months after radical prostatectomy (RP) [4] and 50% by 12 months [5]. This reduced physical capacity leads to prolonged time off work [6], predicts early retirement for those in work before operation [7], and contributes to long-term reductions in health-related quality of life (HRQOL) [3, 5] in these patients. As such, recent work has suggested that measures of surgical success should include return to pre-operative levels of quality of life and physical function [8].
Cardiopulmonary exercise testing (CPET), the gold standard clinical measure of cardiorespiratory fitness (CRF) [9], can help to identify those who are least fit and therefore most at risk of post-operative complications, with the complimentary notion being that if CRF can be improved before surgery then outcomes may also improve. In support of this notion, previous studies in general surgery have deemed the minimal clinically important difference (MCID) to be a pre-operative increase in anaerobic threshold (VO2AT) of 1.5–2.0 ml/kg/min [10, 11]. Indeed, achieving the MCID was associated with a 40% reduction in the odds of post-surgical complications in colorectal patients [10], which could hold true in urological populations also. In addition, higher levels of pre-operative physical activity are related to better HRQOL scores post-operatively [12], with cancer patients who exercise following diagnosis having a lower relative risk of cancer mortality, cancer recurrence and adverse effects from disease and treatment across all cancers [13].
Prehabilitation exercise regimes aim to increase a patient’s physiological reserve before surgery [14] and have been shown to improve functional fitness post operatively [15]. However, within urology most prehabilitation studies have focussed upon reducing specific urological complications (e.g. urinary incontinence) [16] and not on general physiological parameters known to be associated with improved post-operative outcomes and return to pre-operative status such as CRF [14], skeletal muscle mass [17] and body composition [18]. One consideration for all cancer prehabilitation regimes largely regardless of endpoint is that in the UK, the National Cancer Action Team specifies that first treatment (including surgery) should start within 31 days of the decision to treat [19]. This leaves only a short time-window in which to deliver a prehabilitation intervention and elicit change.
In relation to improving CRF, various forms of exercise training have been used in healthy and clinical populations, with moderate continuous (aerobic) training (MCT) the modality most commonly employed. However, most forms of MCT take too long to elicit a beneficial effect on CRF to be useful in patients waiting for surgery for cancer [20]. High-intensity interval training (HIIT) has been proven effective for improving the CRF of both healthy individuals and clinical populations (including certain cancer groups (e.g. lung, bowel and breast)) over shorter time-periods than needed for MCT [20] and as such has potential utility as cancer prehabilitation. Although there are a number of different HIIT protocols, all combine high-intensity exertions with rest periods [21]. Given that time is often cited as a major barrier to exercise training adherence and compliance [22], HIIT regimes with shorter exercise sessions (i.e. low-volume HIIT) may be the most effective in older patient groups.
The primary objective of this study was to investigate whether patients with urological malignancy could achieve the MCID (VO2AT improvement 1.5–2 ml/kg/min) in CRF in response to HIIT, within a window compliant with UK cancer waiting time targets (31 days). Secondary objectives included the effect of HIIT upon blood pressure, body composition, measures of muscle architecture and patient perceived acceptability of the HIIT protocol used.
Subjects/patients and methods
This parallel randomised control trial (1:1 allocation ratio) recruited from August 2016 to June 2018 after ethical approval by the NHS research ethics committee (REC reference: 16/EM/0075, IRAS Project ID 19141). This study was written in accordance with CONSORT guidelines [23] and prospectively registered with Clinical trials.gov (NCT02671617).
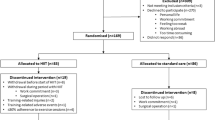
Patients identified at a weekly multidisciplinary team (MDT) meeting, where a decision to operate for urological cancer had been made, were approached by the research team after being informed of their treatment plan in the outpatient clinic. Patients were only approached if the window between MDT decision to treat and their allocated operation date allowed potential for baseline assessment, 10 or more HIIT sessions and reassessment within 72 h before operation. Adherence to the intervention was defined as completing at least ten HIIT sessions.
Patients first received an information sheet and if interested were invited to a study familiarisation The provided written informed consent and underwent medical screening (performed by medically qualified doctor in line with the eligibility criteria for CPET testing defined by the American Thoracic Society [24]). Randomisation was to either the control group (CON; consisting of standard care) or a four week fully-supervised HIIT intervention (HIIT) using a computer-generated list of random permuted block sizes, stratified according to age and gender (to ensure a higher likelihood of equal baseline characteristics). Allocation concealment was ensured by using opaque, sealed envelopes with participant group allocation performed on the first study visit. Due to the nature of the intervention, patients were not blinded to their group assignment, however data collectors were.
At assessment sessions, participants completed the following questionnaires: the Dukes Activity Status Index (DASI) [25], the EuroQol Group 5-level (EQ-5D-5L) [26] and the Warwick Edinburgh Mental Wellbeing Scale (WEMWBS) [27]. Whole-body composition was measured by dual energy X-ray absorptiometry (DXA), after which muscle architecture (muscle thickness, pennation angle and muscle fascicle length) of the vastus lateralis was measured using B-mode ultrasonography [28]. CPET (Lode Corival, Lode, Groningen) was performed as described previously [29], with inline breath-by-breath data collected via a metabolic cart (nSpire Zan 600, Germany), with patients encouraged to exercise to volitional exhaustion [24]. CPET interpretation was conducted by two experienced assessors blinded to time-point (i.e. pre or post-intervention) and group allocation. VO2PEAK (volume of oxygen consumed at the maximal exertion during the test) values were taken as the highest reading in the last 20 s of the test, with VO2AT (volume of oxygen consumed at the anaerobic threshold) determined using a modified V-slope and ventilatory equivalents method [29].
Both HIIT and CON groups were instructed to maintain their habitual physical activity and dietary regimes for the duration of the study. The CON group had assessment sessions 1 and 2 four weeks apart with no visits in between. After assessment session 1, the HIIT group was scheduled for up to 12 HIIT sessions (3–4 times weekly, with no training at weekends) within a 4-week period (i.e. <31 days). The HIIT sessions were delivered on an individual basis at a university exercise laboratory, fully-supervised by a medically qualified doctor. The HIIT protocol was performed on a cycle ergometer and comprised a 2-min warm-up period of unloaded cycling, followed by 5, 1-min exertions at 100–115% of the maximal load (watts (W)) reached during their initial CPET, ending with a 2-min recovery period of unloaded cycling. An increase in wattage was implemented at the mid-way point of training to maintain exercise intensity with progression [29].
Assessment session 2 occurred a maximum of 72 h before surgery for all participants, with participants in the HIIT group also asked to complete a questionnaire about the acceptability of the intervention. This questionnaire has been used previously to assess the acceptability of the same HIIT protocol used in this study [29].
Statistical analysis
An a priori sample size calculation, using data derived from previous work from our group (ANOVA; partial η2 0.15 (effect size 0.42), for a power of 0.80 and α level of 0.05) indicated that 40 patients in total (20 CON group, 20 HIIT group) would be required to detect a difference in VO2AT of 2 ml/kg/min, with the correlation for repeated measures assumed to be 0.7. Including a 20% attrition rate (based on drop-out rates in similar studies) the maximum recruitment number was 48 patients. Descriptive data is presented as mean (±SD), median [inter-quartile range (IQR)] and number (%) as appropriate. To analyse outcomes, we used ANCOVA with pre-intervention baseline values as continuous covariates. Normality was assessed using histograms, and scatter plots were used to assess the relationship between outcomes and covariates. We assessed equality of variance using variance comparison tests. For correlations, we used Pearson’s and Spearman’s as appropriate. Modified intention to treat was conducted on all participants randomised and who underwent both assessment sessions and sensitivity analysis was also performed on prostate cancer patients completing a minimum of ten HIIT sessions. All analyses were conducted using Stata Version 15.1.
Public involvement within the design of this study was during the ethical review process only.
Results
Forty patients were randomised during the study period (Fig. 1). Statistical analysis for the primary outcome measure (VO2AT) was conducted at the end of the ethically approved study period. Baseline characteristics for both groups can be seen in Tables 1 and 2. For those patients randomised to HIIT, adherence (ten or more HIIT sessions) to the exercise training protocol was 84% (16/19). Of those who did not adhere one dropped out of the study, one participant did not attend a single scheduled HIIT session, and one participant only completed six sessions due to a planned holiday. Patients initially trained at 100–115% of maximum wattage achieved at baseline CPET (145 [30] Watts) and all but two patients had a 10% increase in wattage after six sessions as per protocol. The median [IQR] time between baseline and reassessment session for CON and HIIT was 28 [22–29, 31–33] and 30 [27–29, 31] days, respectively. The median [IQR] number of HIIT sessions was 11 [10–12] (excluding those not adherent to the protocol).
Cardiorespiratory fitness
Based on all study participants who completed both pre and post intervention CPET (modified intention to treat analysis), there was a significant improvement in VO2AT (mean difference (MD) 2.26 ml/kg/min (95% CI 1.25 to 3.26)) and VO2PEAK (MD 2.16 ml/kg/min (95% CI 0.24 to 4.08)) following HIIT (Fig. 2). Similarly, there was a significant increase in CPET wattage at failure in the HIIT group (MD 12.86 W (95% CI 5.52–20.19)). In the HIIT group neither VO2PEAK (r2 = 0.02, p = 0.60) nor VO2AT (r2 = 0.04, p = 0.43) improvements were correlated with baseline values. Sensitivity analysis of prostate cancer patients alone (all 15 patients with prostate cancer adhered to the HIIT protocol) showed a significant improvement in VO2AT of 2.19 ml/kg/min (95% CI 1.06–3.32) following HIIT.
Baseline (clear) and post intervention (hatched) cardiorespiratory fitness data for individuals before and after a < 31-day control period (CON; n = 16) or period of high-intensity interval training (HIIT; n = 18). a Volume of oxygen utilised at anaerobic threshold (VO2AT). b Peak volume of oxygen utilisation (VO2PEAK); both measured via cardiopulmonary exercise testing (CPET). *p < 0.05 vs. baseline. Analysis via ANCOVA.
Cardiovascular health
There was a statistically significant reduction in resting blood pressure (BP) parameters following: HIIT (systolic blood pressure (SBP): −8.2 mmHg (95% CI −16.09 to −0.29), diastolic blood pressure (DBP): −6.47 mmHg (95% CI −12.56 to −0.38)) with no change in either parameter in the CON group (Fig. 3)).
Body composition and muscle architecture
Ultrasound image analysis of the m. vastus lateralis, showed significant increases in muscle thickness (MT; MD 0.22 mm (95% CI 0.02 to 0.41)) and pennation angle (PA; MD 2.49 degrees (95% CI 0.42–4.55)) in the HIIT group. Muscle fascicle length (FL) was not altered in either group (HIIT, MD −0.08 mm (95% CI −0.87 to 0.71)). There were no significant changes in any DXA derived parameter of body composition following HIIT (total weight: −0.25 kg (95% CI −1.07 to 0.57)), total body fat percentage: 0.13 % (95% CI −0.64 to 0.90) or total lean mass: −230.9 g (95% CI −1156.10 to 694.16).
Safety and acceptability
There were no adverse safety events reported throughout the study. Mild leg pain at the end of exercise and discomfort from the cycle ergometer seat were reported by two individuals, both of which were self-limiting and required no intervention. All participants were able to achieve >85% maximum predicted heart rate during the HIIT sessions.
There were no significant changes in WEMWBS (−0.88 (95% CI −4.17 to 2.4)), EQ-5D-5L overall health score (visual analogue scale) 3.65 (95% CI −1.97 to 9.28), or 5D-EQ-5L index values (−0.03 (95% CI −0.08 to 0.02)) following HIIT.
Patients in this study reported our HIIT protocol to be enjoyable and highly acceptable, they would recommend HIIT to others, and despite there being no significant changes in the quality of life-based questionnaires, patients were pleased to have improved their own fitness (Table 3). Over half of the participants attended sessions with a spouse or family member who did not participate in the intervention. Specific data sets relating to this results section may be requested from the corresponding author.
Discussion
Patients with urological cancer can make clinically meaningful improvements in objectively measured CRF (VO2AT and VO2PEAK) within 31 days, prior to surgery. As previously shown in healthy volunteers [29] and a different cancer cohort [31], our 5 by 1-min HIIT protocol can be safely delivered, and was reported to be enjoyable by our specific patient group. A number of physiological principles support the implementation of either unimodal or multimodal pre-operative interventions in patients diagnosed with cancer requiring surgical intervention [14] and this study provides evidence for a feasible and efficient unimodal exercise protocol that is effective within urological (predominantly prostate cancer) patients. That we successfully improved CRF within the UK cancer target waiting times of 31 days from decision to treat is important for both feasibility and generalisability, as 93% of urological cancer patients receive first definitive treatment within 31 days of decision to treat (data for the second quarter of 2018) [32]. Individualised supervised laboratory-based HIIT is resource (equipment and staffing) dependent intervention which would likely limit its pragmatic delivery in this format, but does allows monitoring of adherence to the prescribed HIIT sessions and confirmation that high-intensity (>85% heart rate maximum) exercise was achieved during every individual session. Based on previous work, the exercise intensity likely contributed to the significant gains in CRF seen within this study [33], but this intensity and compliance is hard to deliver in unsupervised exercise regimes [30]. We also report significant reductions in BP following just 1-month of HIIT, suggesting HIIT to be more potent than traditional aerobic exercise training where reductions of 5 mmHg in SBP and 3 mmHg in DBP over a 3-month period are reported [34].
Although less objective measures of functional fitness (e.g. improved 6-min walk test (6-MWT) and increased muscle leg power) have been shown to improve within a 31-day timeframe in patients with cancer [35, 36], it has previously proved difficult to improve VOAT, (as measured by CPET) by the MCID (1.5–2.0 ml/kg/min) for all comers in major benign [37] and malignant intra-abdominal surgery [11, 31, 38] using supervised exercise regimes. In addition, until recently, prehabilitation studies in urological patients have largely been aimed at reducing specific urological complications (e.g. urinary incontinence) and this has been successfully achieved with pelvic floor muscle training [16]. However, given the well-reported post-operative decline in physical functioning and reduced HRQOL scores following prostatectomy [3, 5], this study’s findings of improved CRF, reduced BP and positive adaptations in skeletal muscle architecture suggest that our HIIT regime, when used as prehabilitation, has the potential to reduce peri-operative morbidity and mortality, and via pre-operative improvements in strength and fitness, may improve time to return to baseline function after surgery [14, 15].
Although multimodal prehabilitation has shown promise, the efficacy of individual components of these regimes can be difficult to quantify. In addition, exercise prescription as a unimodal intervention has produced mixed outcomes for patients with cancers at different primary sites [11, 20], resulting in a lack of consensus on the optimal training modality and regime. In one urology prehabilitation study, patients awaiting RP who participated in home-based mixed-modality (aerobic exercise, resistance exercise training and pelvic floor training) exercise training did show improvements in 6-MWT before surgery and a more rapid return to pre-operative walk test distances after surgery compared to control [39]. Although this study used different outcome measures to those assessed by us (6-MWT vs. VO2 parameters), it would seem that the presence of prostate cancer does not inhibit positive physiological adaptations to exercise training, adaptations which may be blocked by the presence of other types of cancer [40]. Although it is not entirely clear why patients with certain cancers would be less responsive to HIIT, impaired metabolic processes leading to anabolic blunting (reduced mitochondrial enzyme activity and muscle protein synthetic responses to anabolic stimuli) as seen with colorectal cancer in situ [41] may be less pronounced in prostate cancer. It is hypothesised that HIIT may even mitigate this pro-inflammatory environment induced by cancer [42].
In addition to improvements in CRF, we have also shown favourable changes in muscle architecture of the m. vastus lateralis that are similar in magnitude to those previously seen in healthy older individuals in response to both resistance exercise training [43] and HIIT [29]. Increasing the quality of skeletal muscle (thickness and pennation angle) increases the potential maximal force generating capacity of the muscle [43], which may aid rehabilitation and promote a quicker return to baseline physical function after surgery.
Our HIIT protocol was highly acceptable and enjoyable for the urological cancer patients within this study, although a number of factors outside the exercise regime may have influenced this finding. For example, a number of our patients brought family members or spouses to the HIIT sessions, with previous research reporting quality of life scores in prostate cancer survivors to be significantly associated with the degree of outcome satisfaction among both the patients and their spouses or partners [3]. In addition, our HIIT protocol was delivered in an exercise laboratory environment supervised by a doctor. These factors related to facilities and supervision may also have influenced the self-reported enjoyment of HIIT in this study, and it is as yet unclear how changing elements of our protocol delivery (i.e. unsupervised HIIT) would affect the reported enjoyment and acceptability levels.
Study limitations
The primary limitation of this study relates to the homogeneity of the sample; the majority had prostatic adenocarcinoma, and were white males. Although this specific group of men are clearly trainable, making significant gains in a range of cardiorespiratory parameters comparable to healthy counterparts of a similar age [29], this homogeneity will impact the broader applicability of these findings. Exercise studies are, by their inherent nature, at risk of selection bias as all participants have to be willing to be randomised to the HIIT arm of the study. Although participants were instructed to maintain their habitual activity levels and normal dietary intake throughout the study, we did not measure this in either group, with heightened habitual physical activity a possible contributor to the improvements in CRF seen in the HIIT group. Similarly, alterations in dietary intake may have impacted aspects of this study, especially those related to skeletal muscle mass, given the known importance of contraction × nutrition interactions for optimal anabolism. Referrals to our centre come from a wide geographical area, and eligible patients cited travel time and potential financial cost incurred by travelling from home to the exercise laboratory as barriers to participation. Finally, although post-operative complications (as per the Clavien Dindo classification system (CD) [44]) are commented upon for completeness, the study was not powered for formal analysis of this and as such should be interpreted with caution. Within the HIIT group one patient undergoing radical cystectomy suffered a 4b CD complication due to urosepsis and went on to have an intensive care admission. One patient was readmitted with vomiting and was found to have a port site hernia which was surgically repaired (CD 3b). One patient was transfused red blood cells due to a port site bleed which did not require further intervention (CD 2). Two further patients suffered CD 1 complications including additional analgesia requirement and oral steroids for endotracheal tube irritation. CD complications recorded for the control group were: two patients suffered grade 2 CD complications (IV antibiotic administration due to urosepsis) with a third patient requiring additional analgesia (CD 1).
Concluding statement
Pre-operative HIIT improves CRF, cardiovascular health and measures of skeletal muscle architecture, all of which represent a clinically meaningful and achievable improvement in the health status of urological (primarily prostate) cancer patients in the time available before surgery. Further work is required to investigate the generalisability of this finding to a more heterogenous population, including those with different cancer types. Large randomised trials are required to investigate the effect of prehabilitation upon post-operative complications, socio-economic impact and long-term survival following surgery for urological malignancy.
References
Cancer Research UK. Prostate cancer statistics. Cancer Research UK. 2015. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Four.
Patel HD, Ball MW, Cohen JE, Kates M, Pierorazio P, Allaf ME. Morbidity of urological surgical procedures: an analysis of rates, risk factors, and outcomes. J Urol. 2016;85:552–60.
Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer surviviors. N. Engl J Med. 2008;358:1250–61.
Litwin MS, McGuigan KA, Shpall AI, Dhanani N. Recovery of health related quality of life in the year after radical prostatectomy: early experience. J Urol. 1999;161:515–9. http://www.ncbi.nlm.nih.gov/pubmed/9915438.
Steineck G, Helgesen F, Adolfsson J, Dickman P, Johansson J, Norlén J, et al. Quality of life after radical prostatectomy or watchful waiting. N. Engl J Med. 2002;347:790–6.
Bhalla A, Williams JP, Hurst NG, Speake WJ, Tierney GM, Tou S, et al. One-third of patients fail to return to work 1 year after surgery for colorectal cancer. Tech Coloproctol 2014;18:1153–9.
Tuomi K, Ilmarinen J, Seitsamo J, Huuhtanen P, Martikainen R, Nygård C, et al. Summary of the Finnish research project (1981-1992) to promote the health and work ability of aging workers. Scand J Work Environ Heal. 1997;23:66–71.
Santa Mina D, Matthew AG, Hilton WJ, Au D, Awasthi R, Alibhai SM, et al. Prehabilitation for men undergoing radical prostatectomy: a multi-centre, pilot randomized controlled trial. BMC Surg. 2014;14:89. http://www.biomedcentral.com/content/pdf/1471-2482-14-89.pdf.
Scott JM, Hornsby WE, Lane A, Kenjale AA, Eves ND, Jones LW. Reliability of maximal cardiopulmonary exercise testing in men with prostate cancer. Med Sci Sports Exerc. 2015;47:27–32.
West MA, Lythgoe D, Barben CP, Noble L, Kemp GJ, Jack S, et al. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth. 2014;112:665–71. https://doi.org/10.1093/bja/aet408.
Dunne DFJ, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016;103:504–12.
Santa Mina D, Matthew AG, Hilton WJ, Au D, Awasthi R, Alibhai SMH, et al. Prehabilitation for men undergoing radical prostatectomy: a multi-centre, pilot randomized controlled trial. BMC Surg. 2014;14:89. http://www.biomedcentral.com/content/pdf/1471-2482-14-89.pdf.
Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. 2017;39:71–92.
Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer. Phys Med Rehabil Clin N Am. 2017;28:49–64. https://linkinghub.elsevier.com/retrieve/pii/S1047965116300742.
Minnella EM, Awasthi R, Bousquet-Dion G, Ferreira V, Austin B, Audi C, et al. multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. Eur Urol Focus. 2019. https://www.eu-focus.europeanurology.com/article/S2405-4569(19)30153-1/pdf#.XQIW5ETlrWc.mendeley.
Davie C, Cook T, Rochester P. Pelvic floor muscle training for the management of urinary incontinence following radical prostatectomy. J Assoc Chart Physiother Women’s Heal. 2009;23:4–23.
Jones K, Gordon-Weeks A, Coleman C, Silva M. Radiologically determined sarcopenia predicts morbidity and mortality following abdominal surgery: a systematic review and meta-analysis. World J Surg. 2017;41:2266–79.
Heus C, Bakker N, Verduin WM, Doodeman HJ, Houdijk APJ. Impact of body composition on surgical outcome in rectal cancer patients, a retrospective cohort study. World J Surg. 2019;43:1370–6.
National Cancer Intelligence Network short report, Major resections by cancer site, in England; 2006–2010. Public Health England. Version 2.0, June 2015 (Can be accessed at: http://www.ncin.org.uk/publications/reports).
JEM Blackwell, Doleman B, PJJ Herrod, Ricketts S, Phillips BE, Lund JN, et al. Short-term (8 weeks) high-intensity interval training in diseased cohorts. Med Sci Sport Exerc. 2018;50:1740–9.
Laursen PB, Jenkins DG. The scientific basis for high-intensity interval training. Sports Med. 2002;32:53–73.
Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001.
Pittler MH, Blümle A, Meerpohl JJ, Antes G. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Dtsch Med Wochenschr. 2011;136:20–3. http://www.ncbi.nlm.nih.gov/pubmed/21312153.
American Thoracic S, American College of Chest P. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. http://www.ncbi.nlm.nih.gov/pubmed/12524257.
Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989;64:651–4. http://www.ncbi.nlm.nih.gov/pubmed/2782256.
Van HB, Janssen MF, Feng Y, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L. JVAL. 2012;15:708–15.
Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh mental well-being scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5:63. http://hqlo.biomedcentral.com/articles/10.1186/1477-7525-5-63.
Franchi MV, Atherton PJ, Reeves ND, Fluck M, Williams J, Mitchell WK, et al. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol. 2014;210:642–54. http://www.ncbi.nlm.nih.gov/pubmed/24387247.
Boereboom CL, Phillips BE, Williams JP, Lund JN. A 31-day time to surgery compliant exercise training programme improves aerobic health in the elderly. Tech Coloproctol. 2016;20:375–82. http://link.springer.com/10.1007/s10151-016-1455-1.
Bosworth H. Improving patient treatment adherence: a clinicians guide. 1st ed. Bosworth H, editor. New York: Springer-Verlag; 2010. p. 358.
Boereboom CL, Phillips BE, Williams JP, Lund JN. High intensity interval training does not improve cardiorespiratory fitness within NHS cancer waiting time targets in colorectal cancer patients. Surgical Academic Research Society abstracts 2017. Br J Surg. 2018;105:7–46.
NHS England. Cancer Strategy Implementation Plan. 2018. https://www.england.nhs.uk/cancer/strategy/.
Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–22. http://www.ncbi.nlm.nih.gov/pubmed/20100740.
Herrod PJJ, Doleman B, Blackwell JEM, O’Boyle F, Williams JP, Lund JN, et al. Exercise and other nonpharmacological strategies to reduce blood pressure in older adults: a systematic review and meta-analysis. J Am Soc Hypertens. 2018;12:248–67. https://www.sciencedirect.com/science/article/pii/S1933171118300093.
Chen BP, Awasthi R, Sweet SN, Minnella EM, Bergdahl A, Santa Mina D, et al. Four-week prehabilitation program is sufficient to modify exercise behaviors and improve preoperative functional walking capacity in patients with colorectal cancer. Support Care Cancer. 2017;25:33–40.
Jensen BT, Laustsen S, Jensen JB, Borre M, Petersen AK. Exercise-based pre-habilitation is feasible and effective in radical cystectomy pathways—secondary results from a randomized controlled trial. Support Care Cancer. 2016;24:3325–31.
Tew GA, Batterham AM, Colling K, Gray J, Kerr K, Kothmann E, et al. Randomized feasibility trial of high-intensity interval training before elective abdominal aortic aneurysm repair. Br J Surg. 2017;104:1791–801. http://www.ncbi.nlm.nih.gov/pubmed/28990651.
Banerjee S, Manley K, Shaw B, Kumar V, Ho ETS, Rochester M, et al. ‘Prehabilitation” of patients undergoing radical cystectomy to assist recovery: results of a feasibility study. Eur Urol Suppl. 2015;14:e444.
Santa Mina D, Hilton WJ, Matthew AG, Awasthi R, Bousquet-Dion G, Alibhai SMH, et al. Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol 2018;27:289–98.
Boereboom CL, Blackwell JEM, Williams JP, Phillips BEE, Lund JNN. Short‐term pre‐operative high‐intensity interval training does not improve fitness of colorectal cancer patients. Scand J Med Sci Sports. 2019;1–9. https://doi.org/10.1111/sms.13460.
Phillips BE, Smith K, Liptrot S, Atherton P, Varadhan K, Rennie M, et al. Effect of colon cancer and surgical resection on skeletal muscle mitochondrial enzyme activity in colon cancer patients: a pilot study. J Cachexia Sarcopenia Muscle. 2013;4:71–7.
Papadopoulos E, Santa Mina D. Can we HIIT cancer if we attack inflammation. Cancer Causes Control. 2018;29:7–11. https://doi.org/10.1007/s10552-017-0983-y.
Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Peter Magnusson S, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534:613–23.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg. 2004;240:205–13. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000658-200408000-00003.
D’Amico AV, Whittington R, Bruce Malkowicz S, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Am Med Assoc. 1998;280:969–74. http://jama.ama-assn.org/cgi/content/abstract/280/11/969.
Acknowledgements
We would like to thank Mrs Amanda Gates for her assistance with CPET and HIIT training in this study. We would also like to thank the Urology department at Royal Derby Hospital for their support in completing this study. This research was supported by the MRC Versus Arthritis Centre for Musculoskeletal Ageing Research [grant numbers MR/P021220/1 and MR/R502364/1] and National Institute for Health Research Nottingham Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blackwell, J.E.M., Doleman, B., Boereboom, C.L. et al. High-intensity interval training produces a significant improvement in fitness in less than 31 days before surgery for urological cancer: a randomised control trial. Prostate Cancer Prostatic Dis 23, 696–704 (2020). https://doi.org/10.1038/s41391-020-0219-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-0219-1
This article is cited by
-
Reporting quality of randomized controlled trials in prehabilitation: a scoping review
Perioperative Medicine (2023)
-
Molecular mechanisms underpinning favourable physiological adaptations to exercise prehabilitation for urological cancer surgery
Prostate Cancer and Prostatic Diseases (2023)
-
A systematic review of the impact of postoperative aerobic exercise training in patients undergoing surgery for intra-abdominal cancers
Techniques in Coloproctology (2023)
-
Effects of high-intensity interval training on functional performance and maximal oxygen uptake in comparison with moderate intensity continuous training in cancer patients: a systematic review and meta-analysis
Supportive Care in Cancer (2023)
-
High-Intensity Interval Training and Cardiometabolic Health in the General Population: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Sports Medicine (2023)